Abstract
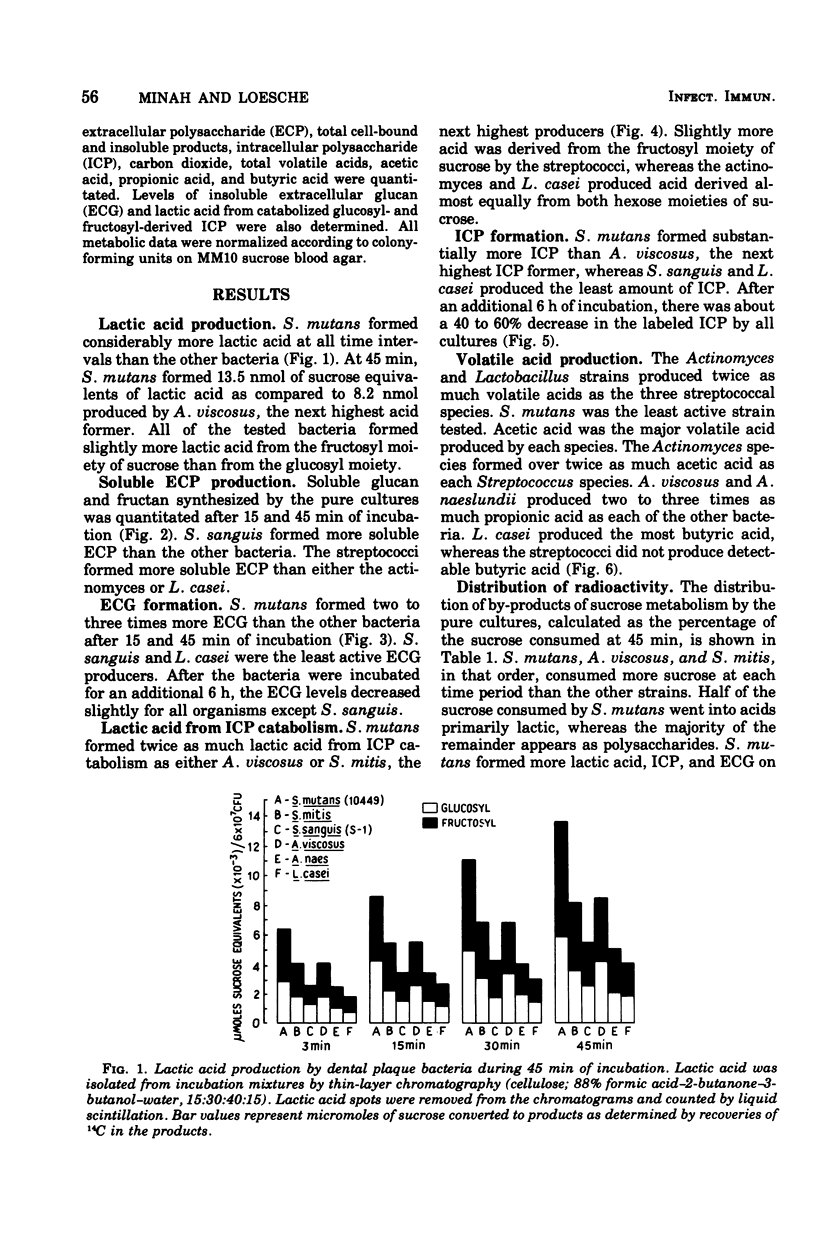
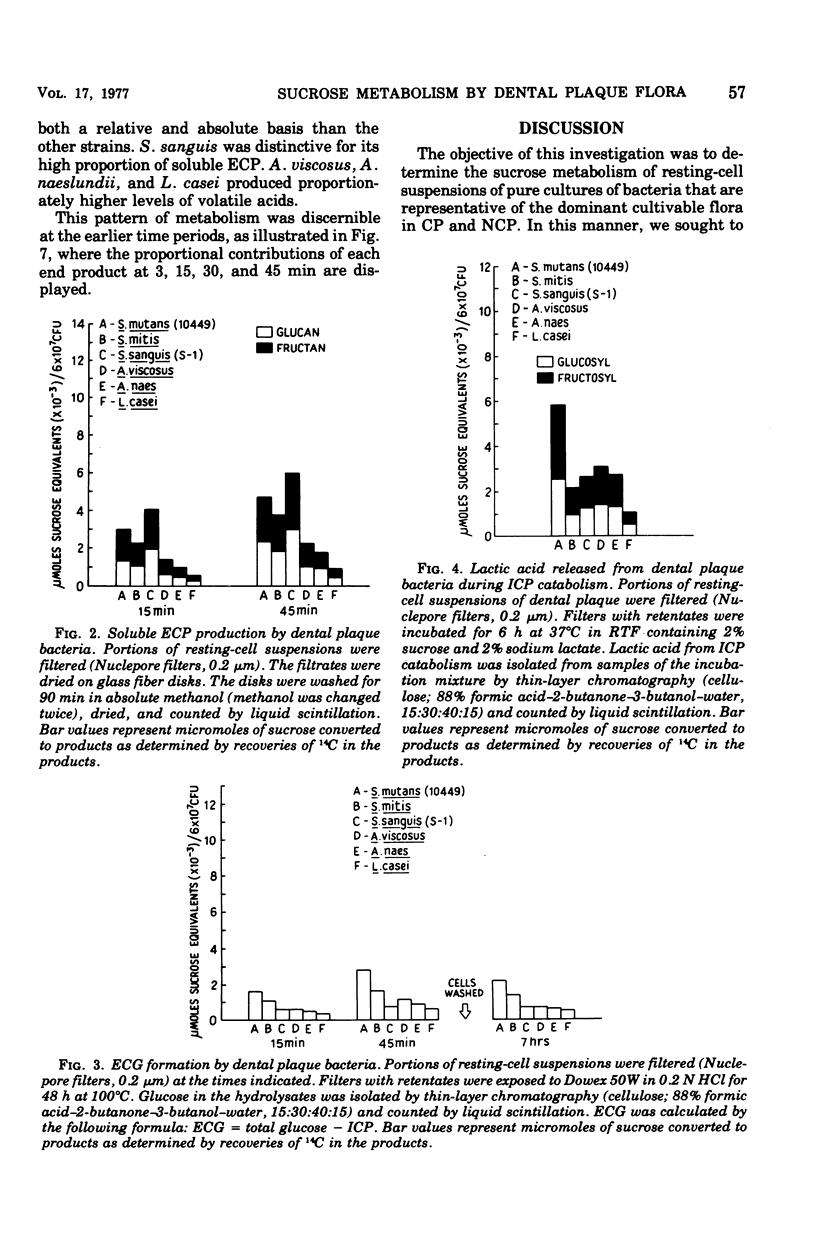
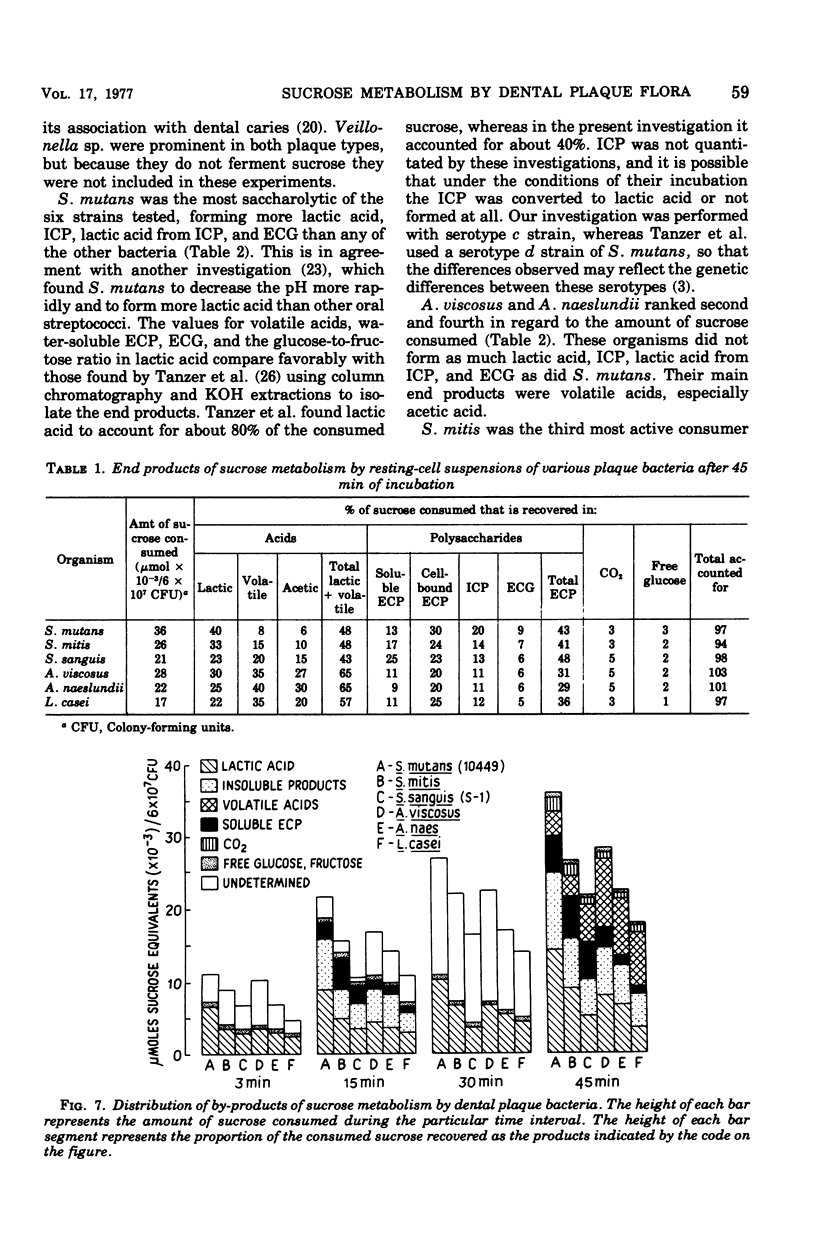
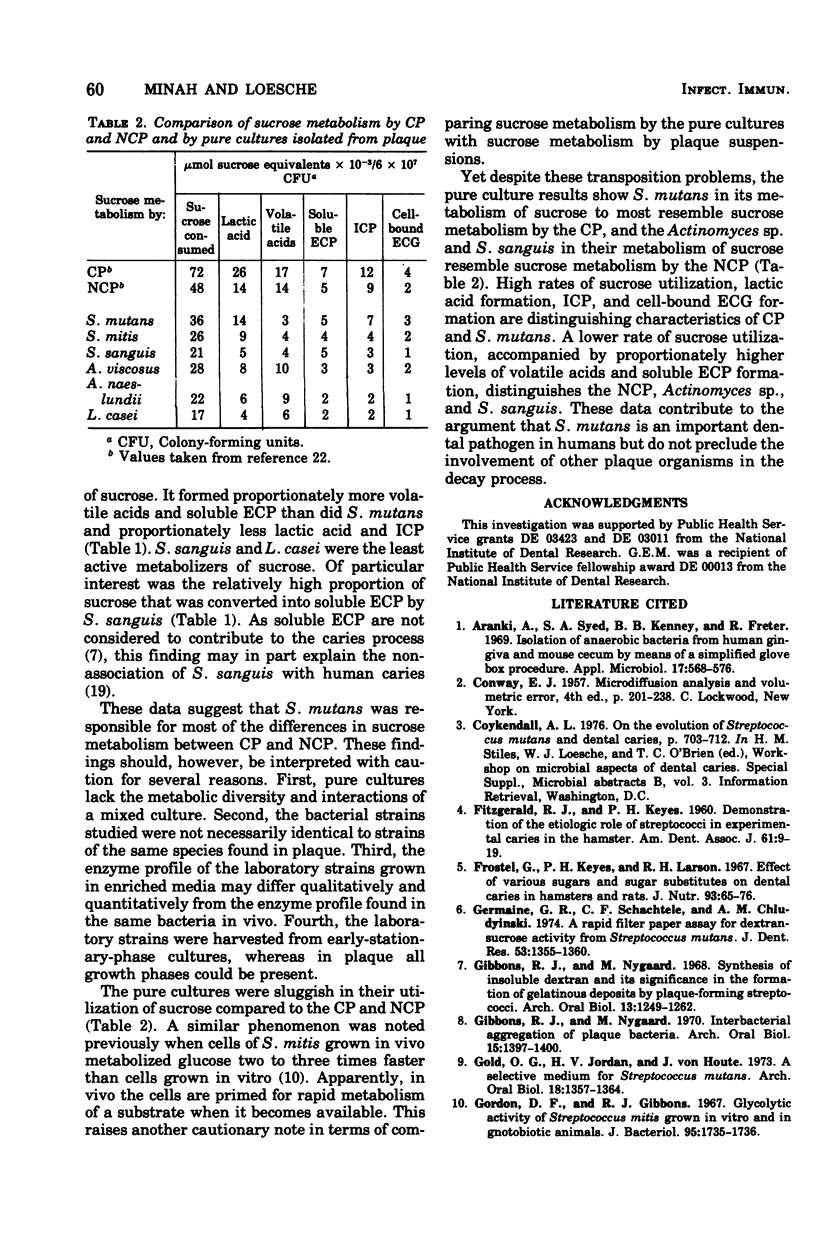
Sucrose metabolism by resting-cell suspensions of pure cultures of representative members of the predominant cultivable flora isolated from cariogenic and non-cariogenic dental plaque was investigated by means of radiochemical techniques. Streptocococcus mutans utilized sucrose at a considerably faster rate than S. sanguis, S. mitis, Actinomyces viscosus, A. naeslundii, or Lactobacillus casei, forming lactic acid, intracellular polysaccharide, insoluble extracellular glucan, and lactic acid from intracellular polysaccharide catabolism at faster rates than the other bacteria. The Actinomyces formed more volatile acids than the streptococci, mostly acetic, and S. sanguis formed more soluble extracellular polysaccharide than the other bacteria. The metabolic activity of S. mutans resembled the pattern of sucrose metabolism of cariogenic plaque, whereas the metabolic activity of the Actinomyces species, the predominant members of non-cariogenic plaque flora, resembled the sucrose metabolism of non-cariogenic plaques.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Frostell G., Keyes P. H., Larson R. H. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J Nutr. 1967 Sep;93(1):65–76. doi: 10.1093/jn/93.1.65. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Gordon D. F., Jr, Gibbons R. J. Glycolytic activity of Streptococcus mitis grown in vitro and in gnotobiotic animals. J Bacteriol. 1967 May;93(5):1735–1736. doi: 10.1128/jb.93.5.1735-1736.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. M., Hartles R. L. The effect of diets contaning different mono- and disaccharides on the incidence of dental caries in the albino rat. Arch Oral Biol. 1969 Mar;14(3):235–241. doi: 10.1016/0003-9969(69)90225-8. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Mühlemann H. R. Modifications of the oral bacterial flora and their influence on dental caries in the rat. I. The effects of inoculating 4 labelled strains of streptococci. Helv Odontol Acta. 1965 Oct;9(2):121–129. [PubMed] [Google Scholar]
- Guggenheim B., Regolati B., Mühlemann H. R. Caries and plaque inhibition by mutanase in rats. Caries Res. 1972;6(4):289–297. doi: 10.1159/000259808. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960 Mar;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- Larson R. H., Theilade E., Fitzgerald R. J. The interaction of diet and microflora in experimental caries in the rat. Arch Oral Biol. 1967 May;12(5):663–668. doi: 10.1016/0003-9969(67)90084-2. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. The predominant cultivable flora of carious plaque and carious dentine. Caries Res. 1973;7(3):201–216. doi: 10.1159/000259844. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism in resting-cell suspensions of caries associated and non-caries-associated dental plaque. Infect Immun. 1977 Jul;17(1):43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onose H., Sandham H. J. pH changes during culture of human dental plaque streptococci on mitis-salivarius agar. Arch Oral Biol. 1976;21(5):291–296. doi: 10.1016/0003-9969(76)90051-0. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J., Simonson L. G. Distribution and frequency of streptococcus mutants in caries-active individuals. J Dent Res. 1972 May-Jun;51(3):882–882. doi: 10.1177/00220345720510034201. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


