Abstract
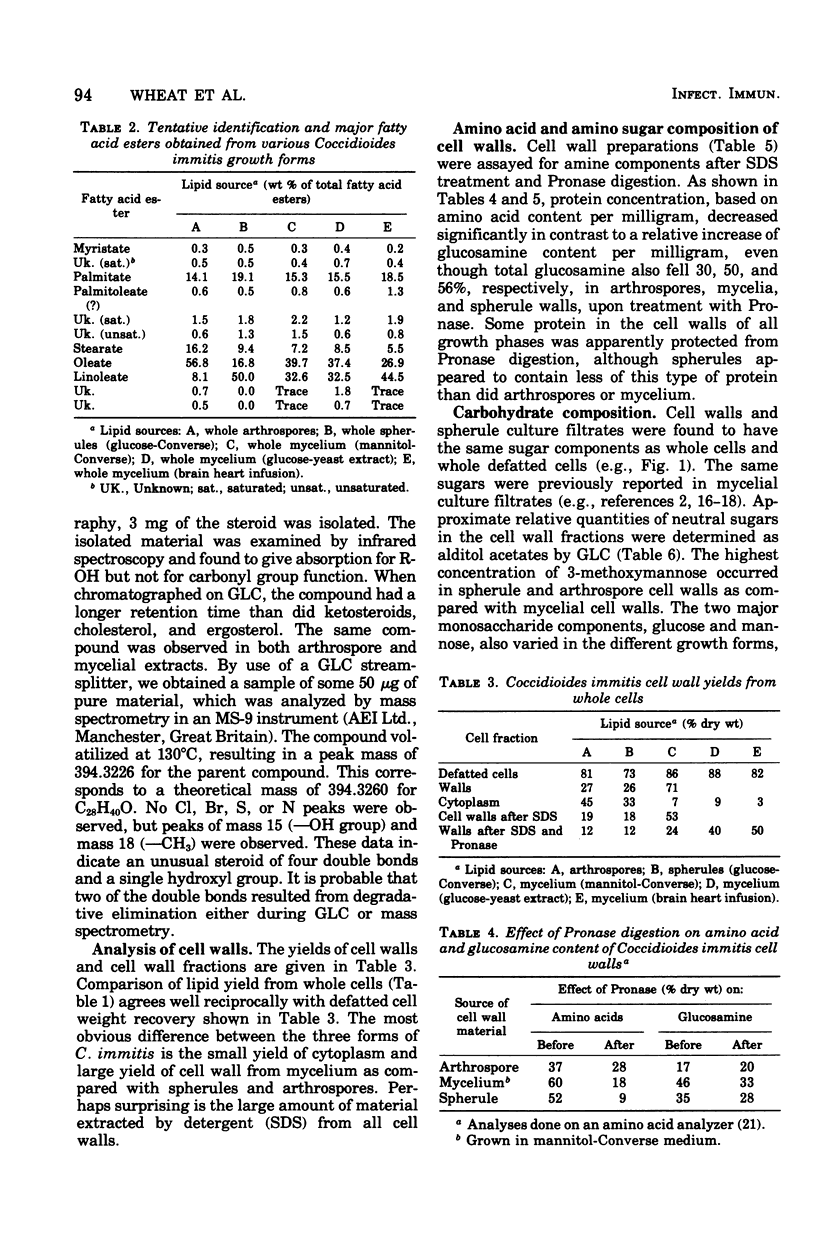
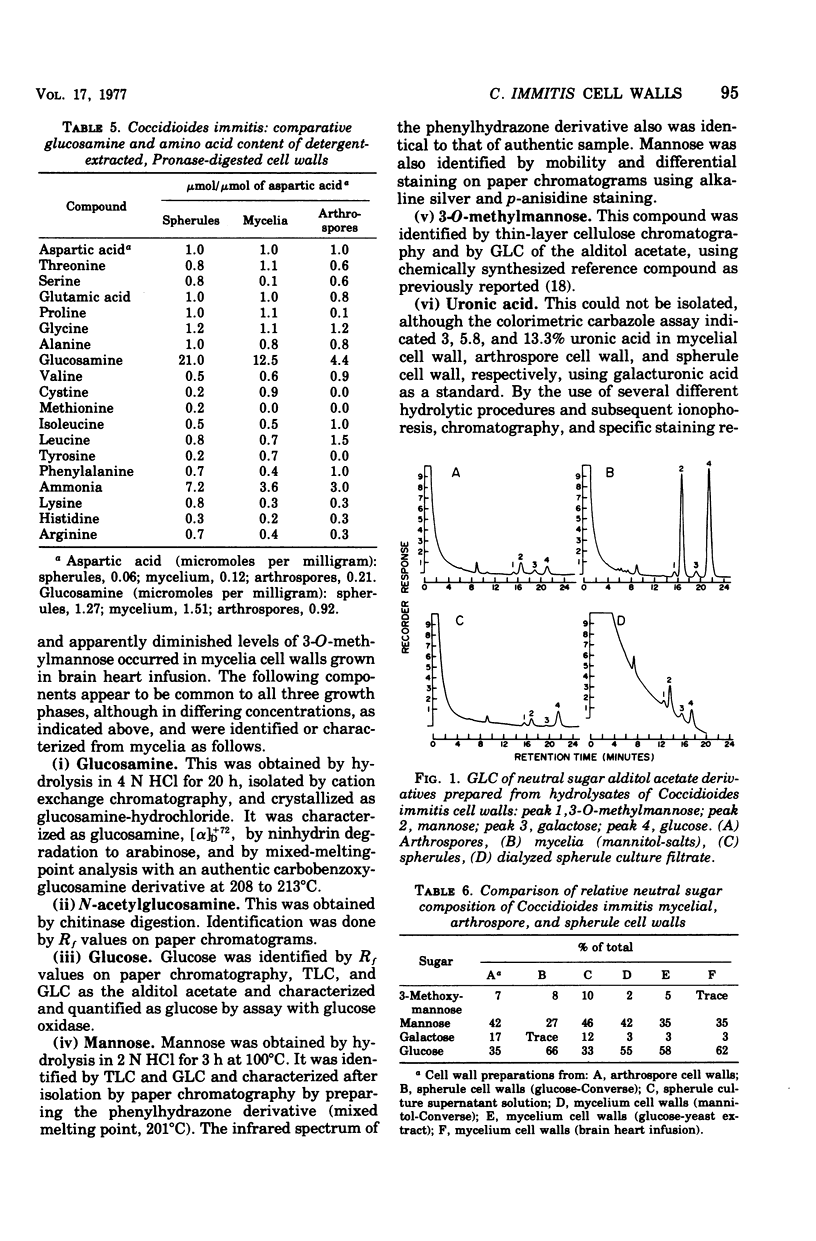
Comparative lipid content, cell wall yield, neutral monosaccharide, glucosamine, and protein (amino acid) contents of arthrospores, mycelia, and spherules of Coccidioides immitis Cash were studied. Cellular lipid contents were found in the decreasing order: spherules, arthrospores, mycelia. Lipid content of mycelia did not reach the level of arthrospores or spherules even when mycelia were grown on relatively rich media. Cell wall yields of spherules were lower than for mycelia when grown on comparable media. Cell walls of arthrospores, mycelia, spherules, and spherule culture filtrate all contained 3-O-methylmannose, mannose, and glucose, but in varying amounts. Cell wall yield and cell wall glucose content increased in mycelia grown in increasingly rich media, whereas mannose content either decreased or remained constant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderes E. A., Finley A. A., Walch H. A. The lipids of an auxutrophic avirulent mutant of Coccidioides immitis. Sabouraudia. 1973 Jul;11(2):149–157. doi: 10.1080/00362177385190311. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BLANK F., BURKE R. C. Chemical composition of the cell wall of Coccidioides immitis. Nature. 1954 May 1;173(4409):829–829. doi: 10.1038/173829a0. [DOI] [PubMed] [Google Scholar]
- CONVERSE J. L. Effect of physico-chemical environment of spherulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1956 Dec;72(6):784–792. doi: 10.1128/jb.72.6.784-792.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONVERSE J. L. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J Bacteriol. 1957 Jul;74(1):106–107. doi: 10.1128/jb.74.1.106-107.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Nutrition of systemic and subcutaneous pathogenic fungi. Bacteriol Rev. 1965 Sep;29(3):406–424. doi: 10.1128/br.29.3.406-424.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Lones G. W., Peacock C. L., McNey F. A. Factors affecting the reversion of Coccidioides immitis spherules to mycelium. Sabouraudia. 1971 Nov;9(3):287–296. [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- Porter J. F., Scheer E. R., Wheat R. W. Characterization of 3-O-methylmannose from Coccidioides immitis. Infect Immun. 1971 Nov;4(5):660–661. doi: 10.1128/iai.4.5.660-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Biochemical challenge of microbial pathogenicity. Bacteriol Rev. 1968 Sep;32(3):164–184. doi: 10.1128/br.32.3.164-184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. S., Brendel K., Scheer E., Wheat R. W. Ion-exchange separation and automated assay of complex mixtures of amino acids and hexosamines. Anal Biochem. 1970 Mar;34:206–225. doi: 10.1016/0003-2697(70)90101-6. [DOI] [PubMed] [Google Scholar]
- TARBET J. E., BRESLAU A. M. Histochemical investigation of the spherule of Coccidioides immitis in relation to host reaction. J Infect Dis. 1953 Mar-Apr;92(2):183–190. doi: 10.1093/infdis/92.2.183. [DOI] [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R., Scheer E. Cell walls of Coccidioides immitis: neutral sugars of aqueous alkaline extract polymers. Infect Immun. 1977 Jan;15(1):340–341. doi: 10.1128/iai.15.1.340-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


