Abstract
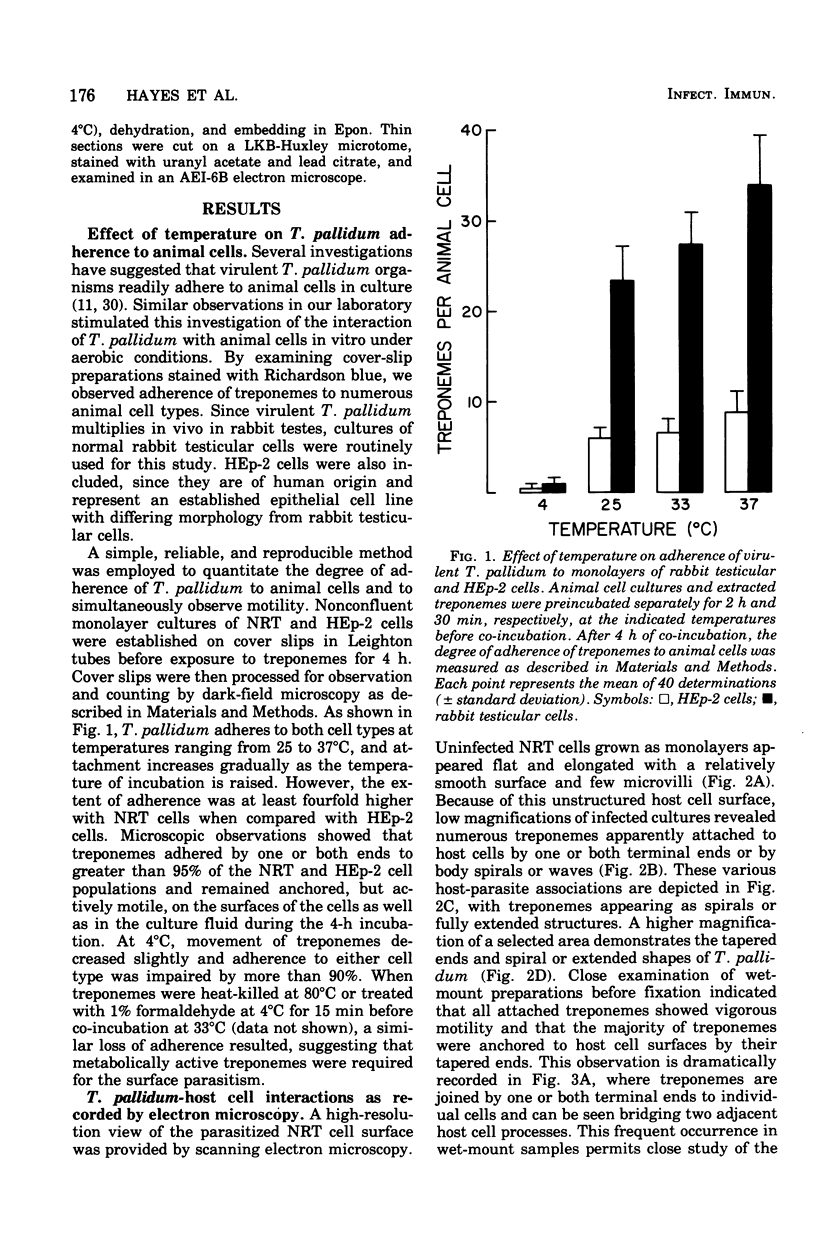
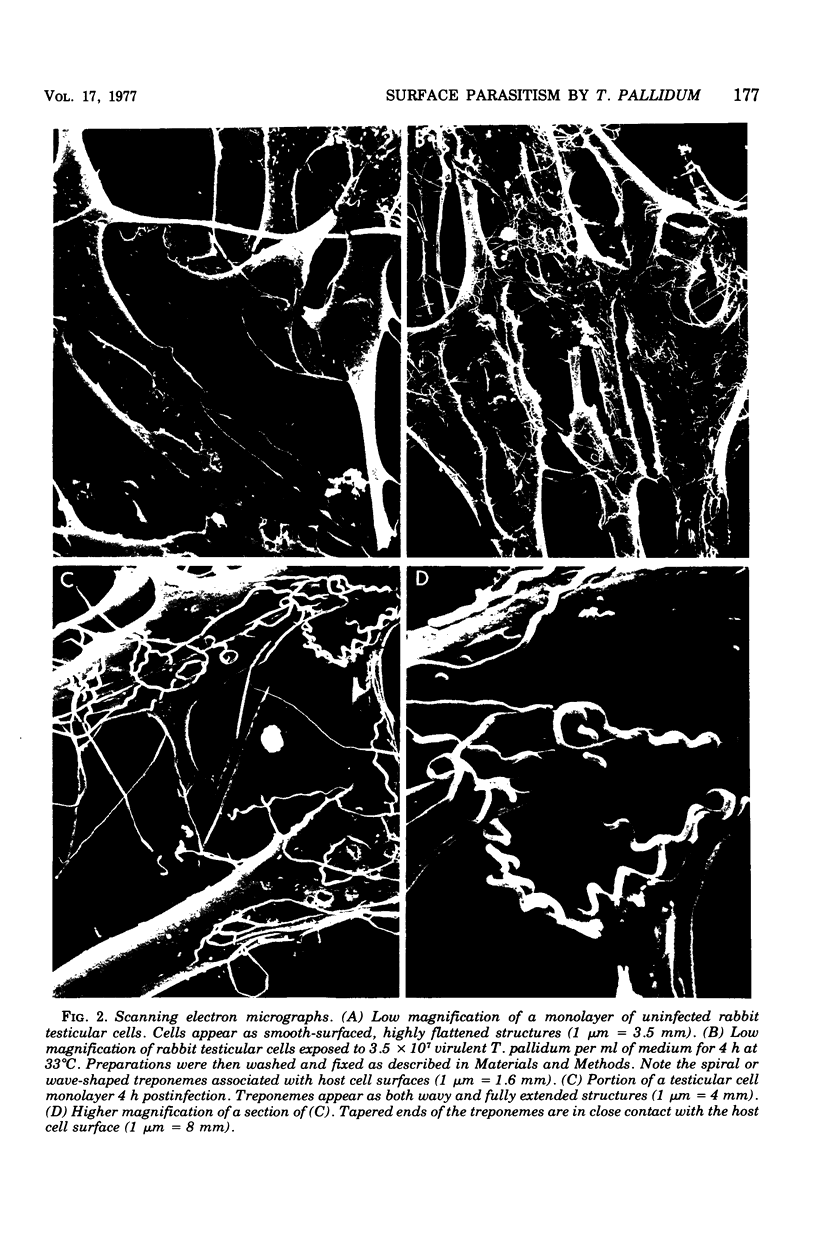
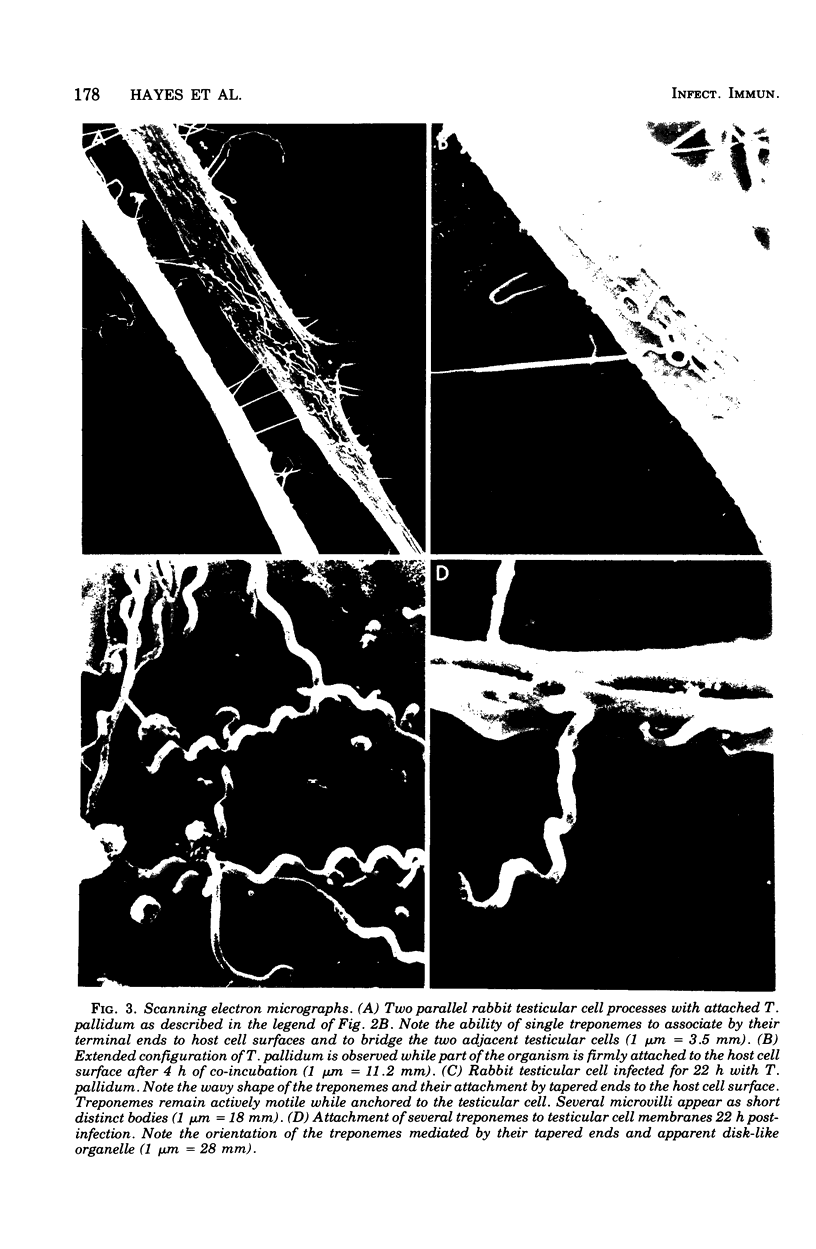
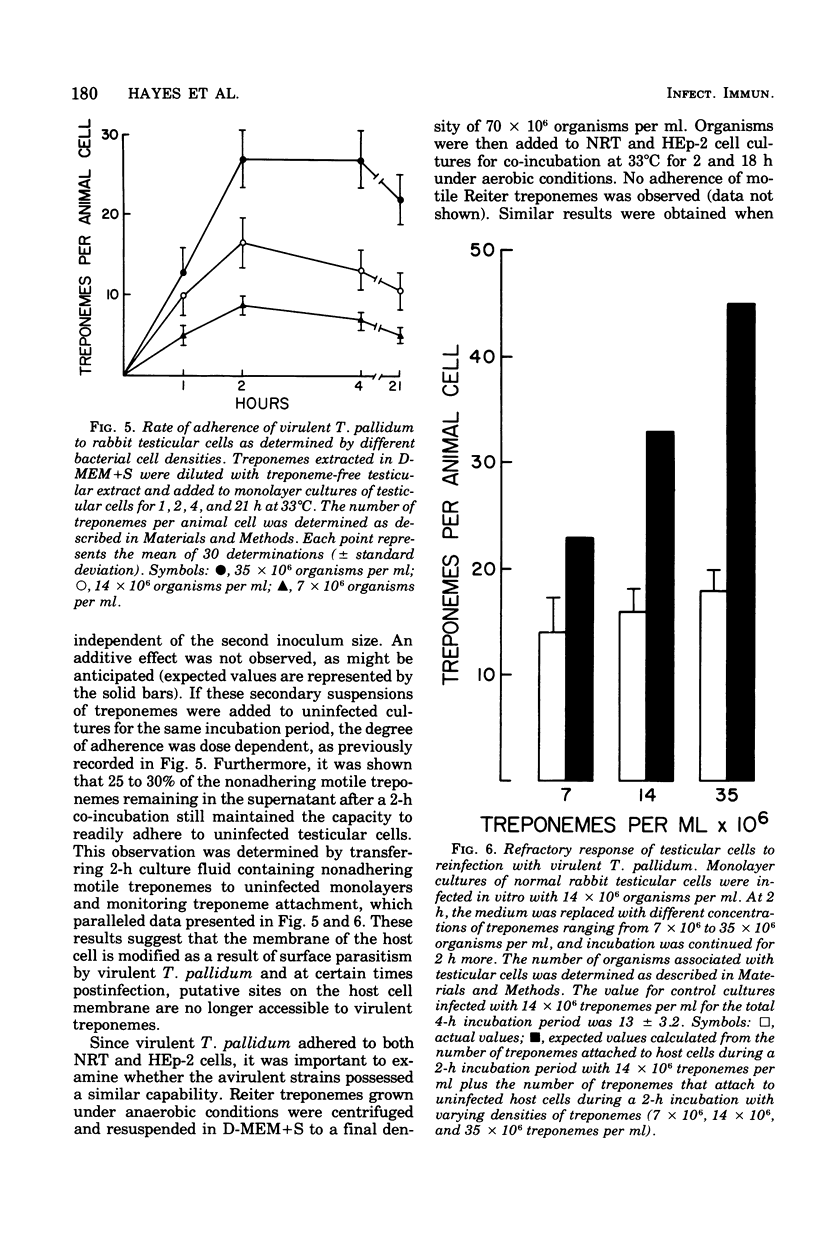
The interaction between virulent Treponema pallidum extracted from infected rabbit testes and animal cells in culture was examined. The extent of treponemal attachment to monolayers of normal rabbit testicular and HEp-2 cells was dependent upon the incubation temperature and retained motility of the spirochetes. The specific orientation of treponemes to host cell surfaces was demonstrated by dark-field microscopic examination of wet-mount preparations and scanning and transmission electron microscopy. Once attached, T. pallidum organisms remained actively motile yet anchored in place by their terminal tapered structures. After several hours of co-incubation, maximal attachment was attained, and the degree of parasitism seemed regulated not only by available surface sites on individual host cells but also by the proposed membrane response of parasitized cells to continued exposure to treponemes. The avirulent strain, Treponema phagedenis biotype Reiter, did not adhere to monolayer cultures. Characterization of host cell determinants that permitted surface colonization by T. pallidum was attempted. Also, properties of virulent treponemes that enabled surface parasitism were monitored by measuring the effects of enzymes, detergents, and metabolic inhibitors on the host-parasite interaction. Results reinforced the specific nature of the treponemal attachment mechanism. Furthermore, the ability of convalescent rabbit sera to reduce attachment of treponemes to host cells suggested that surface structures on T. pallidum could be masked or inactivated by host components, thus providing a potentially effective research approach for investigating the pathogenesis of syphilis and screening appropriate vaccine candidates.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuckle J. B. The attachment of Clostridium welchii (Cl. perfringens) type C to intestinal villi of pigs. J Pathol. 1972 Feb;106(2):65–72. doi: 10.1002/path.1711060202. [DOI] [PubMed] [Google Scholar]
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Hayes N. C. Virulent Treponema pallidum: aerobe or anaerobe. Infect Immun. 1976 Mar;13(3):704–711. doi: 10.1128/iai.13.3.704-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Ukena T. E., Yin H. H. The cell surface. N Engl J Med. 1975 Mar 6;292(10):515–520. doi: 10.1056/NEJM197503062921007. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Barber M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 1974 Jul;10(1):123–127. doi: 10.1128/iai.10.1.123-127.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Shape of Treponema pallidum. J Bacteriol. 1972 Feb;109(2):943–944. doi: 10.1128/jb.109.2.943-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Specific agglutination of Treponema pallidum by sera from rabbits and human beings with treponemal infections. J Exp Med. 1955 Apr 1;101(4):367–382. doi: 10.1084/jem.101.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzat N. N., Knox J. M., Dacres W. G., Smith E. B. Resistance and serological changes in rabbits immunized with virulent Treponema pallidum sonicate. Acta Derm Venereol. 1971;51(2):157–160. [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENT J. F., DE WEERDT J. B. Enhancement by lysozyme of the sensitivity of Treponema pallidum immobilization tests. Br J Vener Dis. 1963 Mar;39:37–40. doi: 10.1136/sti.39.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER M., HARDY P. H., Jr, NELL E. E. Influence of lysozyme upon the treponeme immobilization reaction. Am J Hyg. 1961 Mar;73:236–244. doi: 10.1093/oxfordjournals.aje.a120182. [DOI] [PubMed] [Google Scholar]
- Metzger M., Smogór W. Artificial immunization of rabbits against syphilis. I. Effect of increasing doses of treponemes given by the intramuscular route. Br J Vener Dis. 1969 Dec;45(4):308–312. doi: 10.1136/sti.45.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Poste G. The cancer cell: dynamic aspects and modifications in cell-surface organization (first of two parts). N Engl J Med. 1976 Jul 22;295(4):197–203. doi: 10.1056/NEJM197607222950405. [DOI] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Further studies of the morphology of Treponema pallidum under the electron microscope. Br J Vener Dis. 1969 Jun;45(2):87–116. doi: 10.1136/sti.45.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEQUEIRA P. J. The morphology of Treponema pallidum. Lancet. 1956 Oct 13;271(6946):749–749. doi: 10.1016/s0140-6736(56)90959-x. [DOI] [PubMed] [Google Scholar]
- SWAIN R. H. Electron microscopic studies of the morphology of pathogenic spirochaetes. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):117–128. doi: 10.1002/path.1700690117. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- Wiegand S. E., Strobel P. L., Glassman L. H. Electron microscopic anatomy of pathogenic Treponema pallidum. J Invest Dermatol. 1972 Apr;58(4):186–204. doi: 10.1111/1523-1747.ep12539907. [DOI] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]





