Abstract
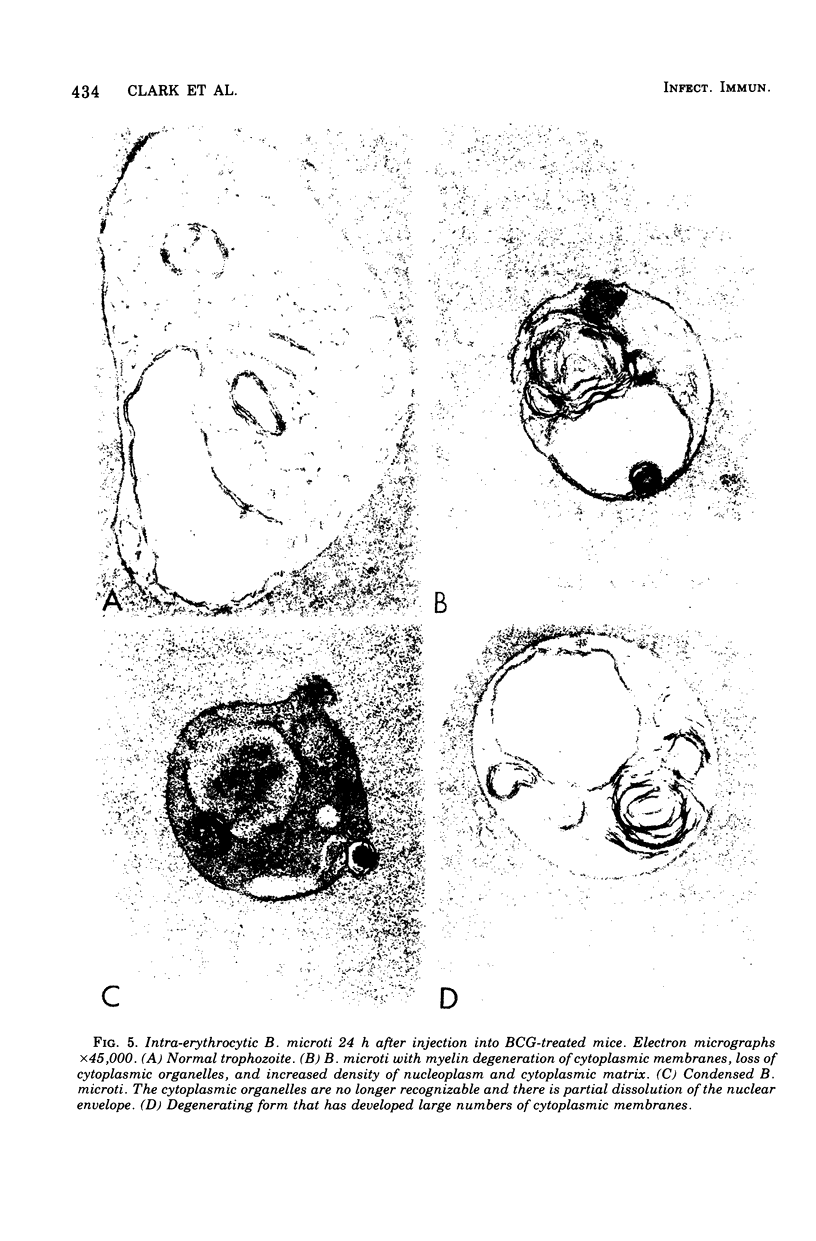
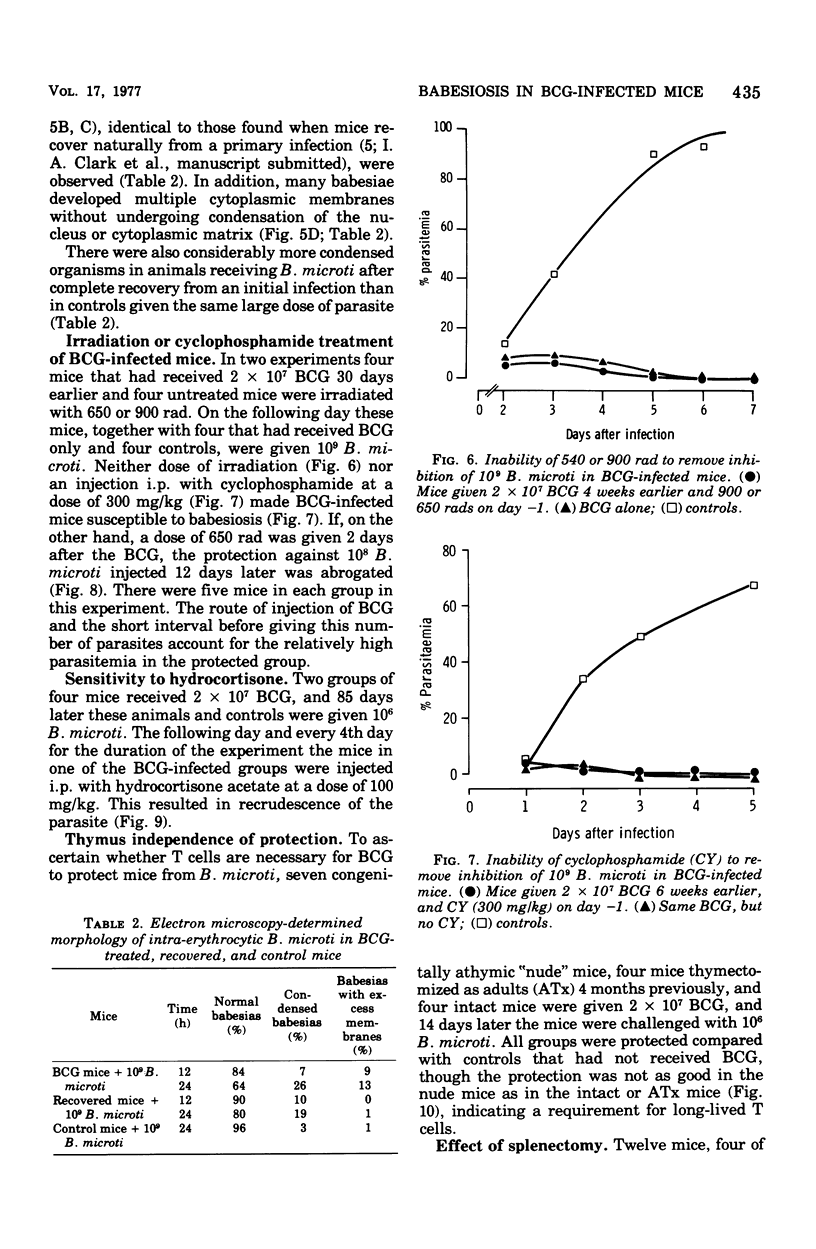
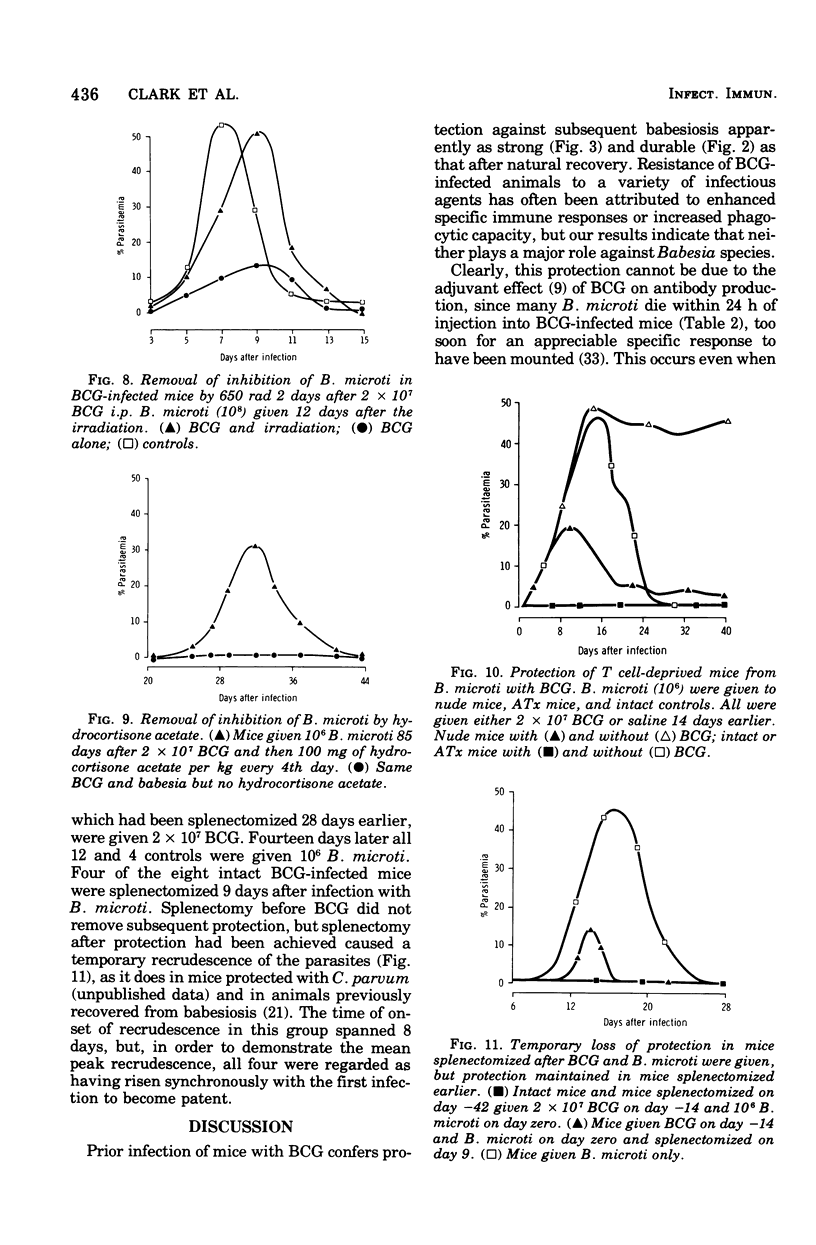
Infection of mice with Bacillus Calmette-Guérin (BCG) provided good protection against Babesia species. The intensity and duration of this protection was similar to that established after natural recovery from babesiosis. It developed too soon after the first exposure to the parasite, and was too radioresistant, to be attributable to specific antibody production. In addition, the parasites degenerated within circulating erythrocytes. This phenomenon is inconsistent with phagocytosis or lysis of parasites or parasitized cells, or prevention of entry of parasites into erythrocytes, causing the observed protection. Hence the phenomenon is best explained by the release of a nonspecific mediator that can limit multiplication of parasites within erythrocytes. These results not only throw light on mechanisms of immunity against hemoprotozoa. There are many points of similarity between the nonspecific protection BCG and Corynebactium parvum provide against Babesia species and inhibition of tumor growth by these agents. Therefore, babesiosis in mice may be a convenient experimental model for assessing stimulation of the mononuclear phagocyte system, which appears to be the basis of nonspecific immunity against bacteria, parasites, and tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., GRUMBACH F., HALPERN B. N., LEVADITI J., RIST N. Etude de l'activité granulopexique du système réticulo-endothélial au cours de l'infection tuberculeuse expérimentale de la souris. Ann Inst Pasteur (Paris) 1954 Sep;87(3):291–300. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Richmond J. E., Wills E. J., Allison A. C. Immunity to intra-erythrocytic protoza. Lancet. 1975 Dec 6;2(7945):1128–1129. doi: 10.1016/s0140-6736(75)91010-7. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. The effect of Mycobacterium tuberculosis (BCG) infection on the resistance of mice to bacterial endotoxin and Salmonella enteritidis infection. Br J Exp Pathol. 1959 Jun;40(3):281–290. [PMC free article] [PubMed] [Google Scholar]
- Healy G. R., Speilman A., Gleason N. Human babesiosis: reservoir in infection on Nantucket Island. Science. 1976 Apr 30;192(4238):479–480. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Lambert L. H., Jr, Remington J. S. Effects of Corynebacterium parvum treatment and Toxoplasma gondii infection on macrophage-mediated cytostasis of tumour target cells. Immunology. 1976 Dec;31(6):837–846. [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek C., Chalvet H., Stiffel C. L., Biozzi G. Study of the mechanism of Corynebacterium parvum anti-tumour activity. I. Protective effect on the growth of two syngeneic tumours. Int J Cancer. 1976 Apr 15;17(4):511–517. doi: 10.1002/ijc.2910170414. [DOI] [PubMed] [Google Scholar]
- Milas L., Hunter N., Withers H. R. Corynebacterium-induced protection against artificial pulmonary metastases of a syngeneic fibrosarcoma in mice. Cancer Res. 1974 Mar;34(3):613–620. [PubMed] [Google Scholar]
- Minden P., Sandridge J., Wainberg M. A., McClatchy J. K. Transmission of BCG-associated tumor resistance from maternal to newborn guinea pigs. J Natl Cancer Inst. 1976 Jan;56(1):153–157. doi: 10.1093/jnci/56.1.153. [DOI] [PubMed] [Google Scholar]
- Purnell D. M., Otterstrom J. R., Bartlett G. L., Kreider J. W. Antitumor activity of killed Corynebacterium parvum suspensions in a murine mammary adenocarcinoma CaD2) system. J Natl Cancer Inst. 1976 Jun;56(6):1171–1175. doi: 10.1093/jnci/56.6.1171. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinska M. A. Ultrastructure of intraerythrocytic Babesia microti with emphasis on the feeding mechanism. J Protozool. 1976 May;23(2):224–233. doi: 10.1111/j.1550-7408.1976.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Nishio J., Shonnard J. T. Two new inhibitory activities in blood of mice with delayed hypersensitivity, after challenge with specific antigen. Infect Immun. 1974 Apr;9(4):631–635. doi: 10.1128/iai.9.4.631-635.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Ribi E., Granger D. L., Youngner J. S. Migration inhibitory factor and type II interferon in the circulation of mice sensitized with mycobacterial components. J Immunol. 1975 Jan;114(1 Pt 2):354–359. [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Nishio J., Neta R. Tumor suppression by a lymphokine released into the circulation of mice with delayed hypersensitivity. J Natl Cancer Inst. 1975 Nov;55(5):1233–1236. doi: 10.1093/jnci/55.5.1233. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as a therapeutic antitumor agent in mice. I. Systemic effects from intravenous injection. J Natl Cancer Inst. 1974 Sep;53(3):855–860. doi: 10.1093/jnci/53.3.855. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as an immunotherapeutic anticancer agent. Semin Oncol. 1974 Dec;1(4):367–378. [PubMed] [Google Scholar]
- Scott M. T. In vivo cortisone sensitivity of nonspecific antitumor activity of Corynebacterium parvum-activated mouse peritoneal macrophages. J Natl Cancer Inst. 1975 Mar;54(3):789–792. doi: 10.1093/jnci/54.3.789. [DOI] [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W. Salmonellosis in Mice Infected with Mycobacterium bovis BCG II. Resistance to Infection. Infect Immun. 1970 Jun;1(6):583–586. doi: 10.1128/iai.1.6.583-586.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALIAFERRO W. H., TALIAFERRO L. G., JANSSEN E. F. The localization of x-ray injury to the initial phases of antibody response. J Infect Dis. 1952 Sep-Oct;91(2):105–124. doi: 10.1093/infdis/91.2.105. [DOI] [PubMed] [Google Scholar]
- Tabbara K. J., O'Connor G. R., Nozik R. A. Effect of immunization with attenuated Mycobacterium bovis on experimental toxoplasmic retinochoroiditis. Am J Ophthalmol. 1975 Apr;79(4):641–647. doi: 10.1016/0002-9394(75)90804-1. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Finkelstein M. S. The kinetics of antibody formation. Prog Allergy. 1967;10:37–83. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M., Dunbar N., Ghaffar A. The growth of tumours in T-cell deprived mice and their response to treatment with Corynebacterium parvum. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):97–102. doi: 10.1098/rspb.1973.0034. [DOI] [PubMed] [Google Scholar]