Abstract
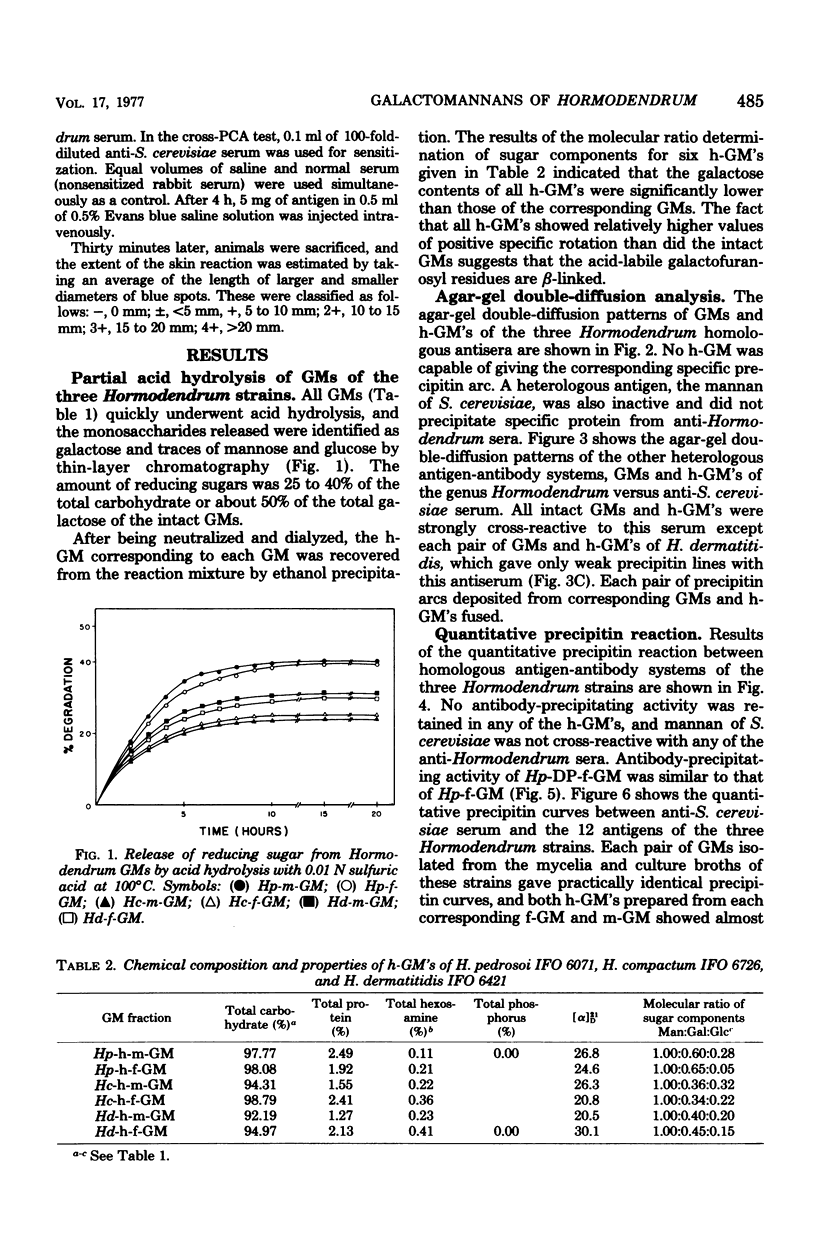
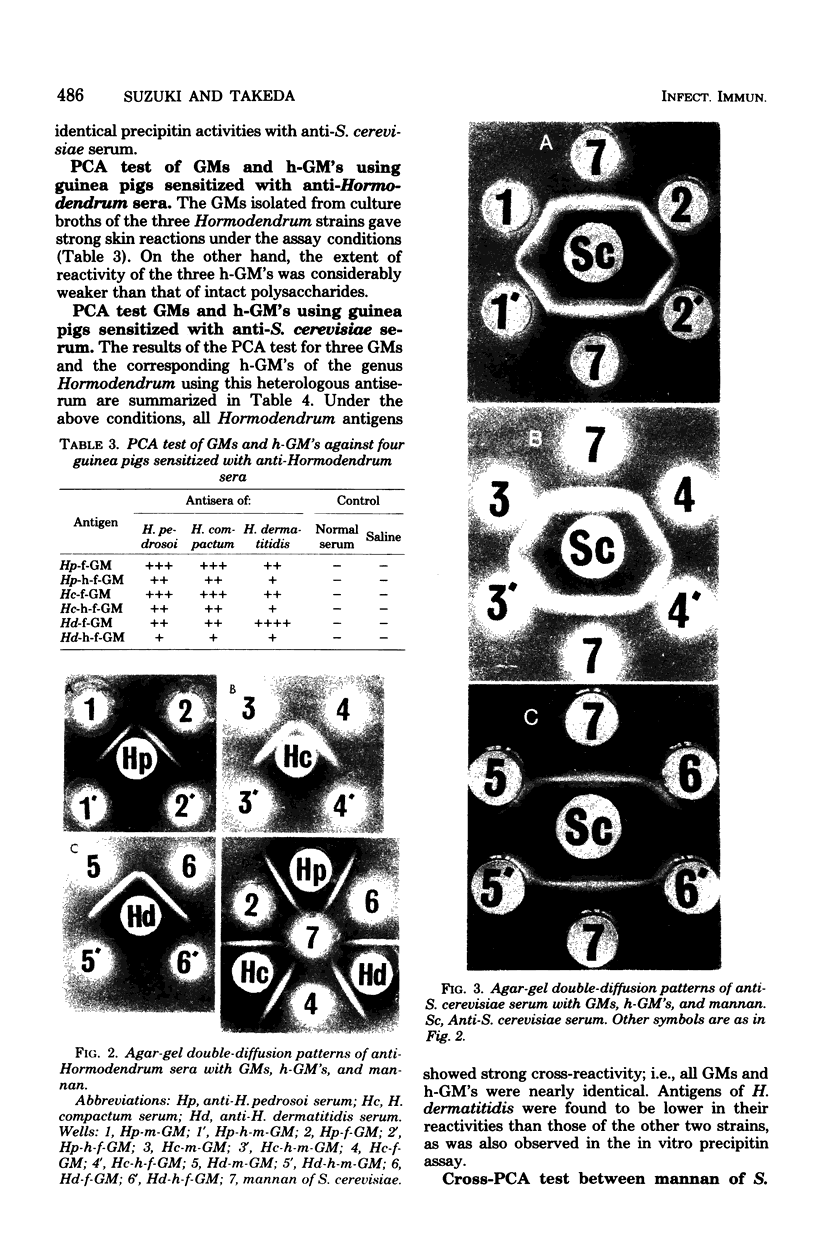
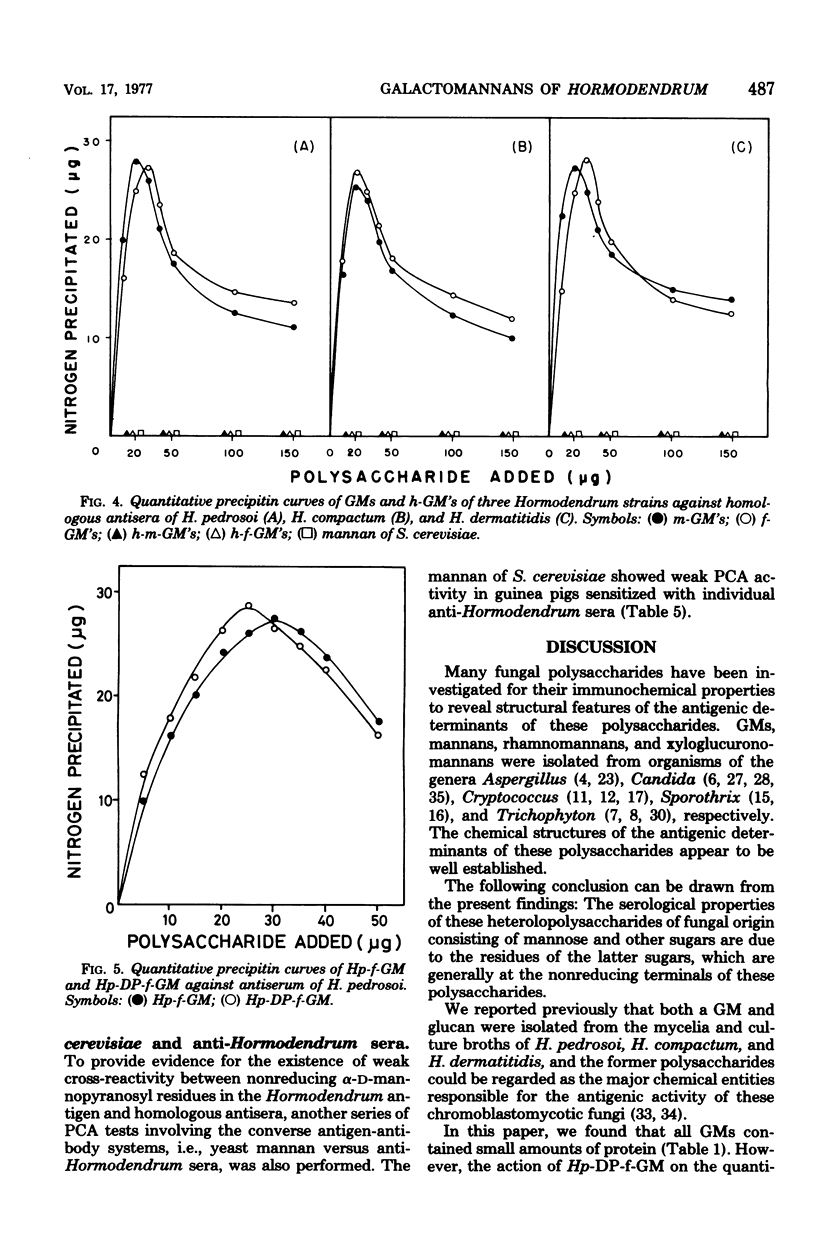
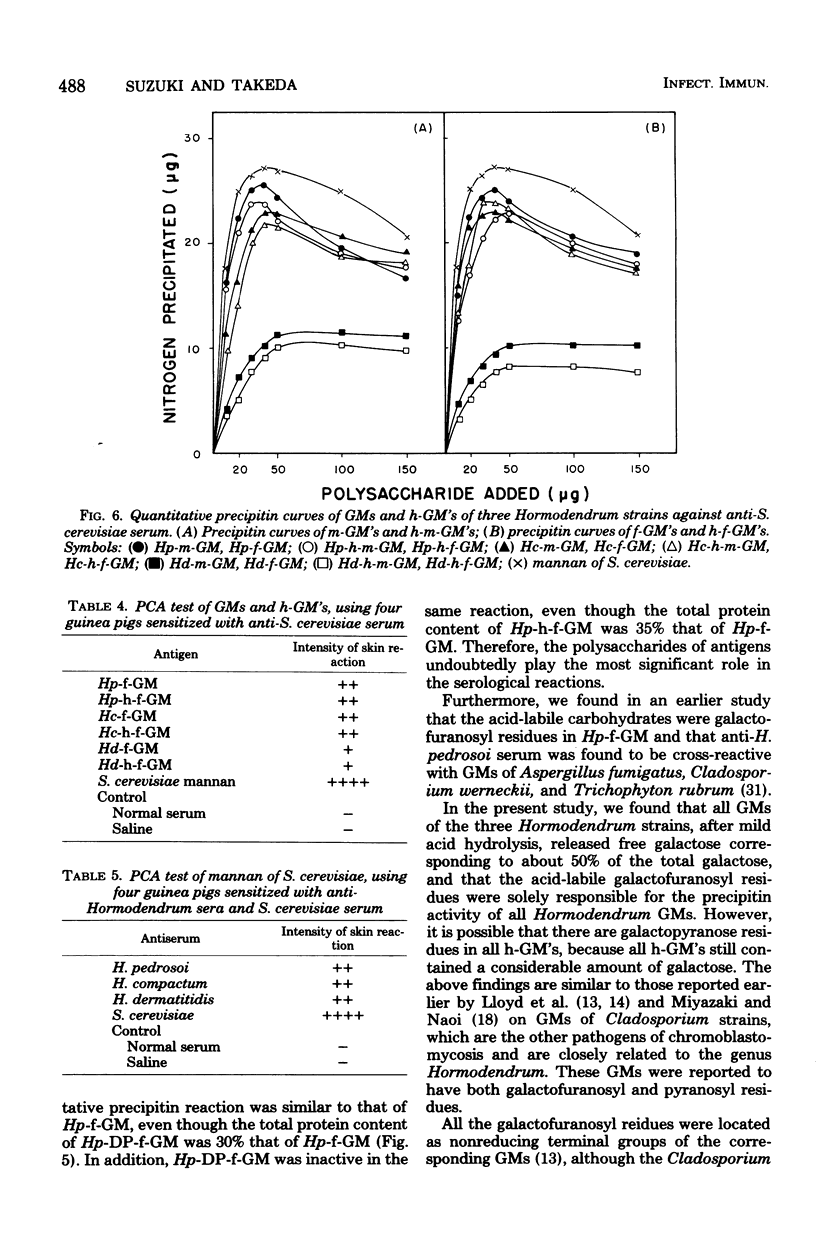
Six major antigenic galactomannans isolated from both mycelia and culture broths of three representative strains of three species of the genus Hormodendrum, H. pedrosoi IFO 6071, H. compactum IFO 6726, and H. dermatitidis IFO 6421, were investigated for their immunochemical properties by precipitin reaction and the passive cutaneous anaphylaxis test. We found that partial acid hydrolysis of the these galactomannans with 0.005 M sulfuric acid yielded free galactose and corresponding acid-resistant core moieties. In agar-gel double-diffusion reactions, no acid-resistant core moiety of galactomannan showed a corresponding precipitin line against homologous antiserum, indicating that the acid-labile galactofuranosyl residues were solely responsible for the precipitin activity of these galactomannans. All galactomannans and their core moieties gave sharp cross-precipitin lines against anti-Saccharomyces cerevisiae serum, specific for the alpha1 leads to 3- or alpha1 leads to 2-linked D-mannopyranosyl residue. Quantitative precipitin reactions of the same antigen-antibody systems showed that all acid-resistant core moieties were completely inactive in the homologous systems but were highly cross-reactive with anti-S. cerevisiae serum. Passive cutaneous anaphylaxis tests using guinea pigs sensitized with homologous antisera or with anti-S. cerevisiae serum provided results consistent with those obtained in the in vitro assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BARKER S. A., CRUICKSHANK C. N., MORRIS J. H., WOOD S. R. The isolation of trichophytin glycopeptide and its structure in relation to the immediate and delayed reactions. Immunology. 1962 Nov;5:627–632. [PMC free article] [PubMed] [Google Scholar]
- EGGSTEIN M., KREUTZ F. H. Vergleichende Untersuchungen zur quantitativen Eiweissbestimmung im Liquor und eiweissarmen Lösungen. Klin Wochenschr. 1955 Oct 1;33(37-38):879–884. doi: 10.1007/BF01473099. [DOI] [PubMed] [Google Scholar]
- EINBINDER J. M., BENHAM R. W., NELSON C. T. Chemical analysis of the capsular substance of Cryptococcus neoformans. J Invest Dermatol. 1954 Apr;22(4):279–283. doi: 10.1038/jid.1954.40. [DOI] [PubMed] [Google Scholar]
- EVANS E. E., KESSEL J. F. The antigenic composition of Cryptococcus neoformans. II. Serologic studies with the capsular polysaccharide. J Immunol. 1951 Aug;67(2):109–114. [PubMed] [Google Scholar]
- Lloyd K. O., Bitoon M. A. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. Structural and immunochemical studies. J Immunol. 1971 Sep;107(3):663–671. [PubMed] [Google Scholar]
- Lloyd K. O. Isolation, characterization, and partial structure of peptido galactomannans from the yeast form of Cladosporium werneckii. Biochemistry. 1970 Aug 18;9(17):3446–3453. doi: 10.1021/bi00819a025. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O. Molecular organization of a covalent peptido-phospho-polysaccharide complex from the yeast form of Cladosporium werneckii. Biochemistry. 1972 Oct 10;11(21):3884–3890. doi: 10.1021/bi00771a008. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Travassos L. R. Immunochemical studies on L-rhamno-D-mannans of Sporothrix schenckii and related fungi by use of rabbit and human antisera. Carbohydr Res. 1975 Mar;40(1):89–97. doi: 10.1016/s0008-6215(00)82671-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Naoi Y. Extracellular polysaccharide of Cladosporium herbarum. Studies on fungal polysaccharide. XIII. Chem Pharm Bull (Tokyo) 1974 Jun;22(6):1360–1365. doi: 10.1248/cpb.22.1360. [DOI] [PubMed] [Google Scholar]
- OVARY Z. Immediate reactions in the skin of experimental animals provoked by antibody-antigen interaction. Prog Allergy. 1958;5:459–508. [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi O., Yokota K., Suzuki M. Immunochemical and biochemical studies of fungi. 13. On the galactomannans isolated from mycelia and culture filtrates of several filamentous fungi. Jpn J Microbiol. 1969 Mar;13(1):1–7. [PubMed] [Google Scholar]
- Shimonaka H., Noguchi T., Kawai K., Hasegawa I., Nozawa Y., Ito Y. Immunochemical studies on the human pathogen Sporothrix schenckii: effects of chemical and enzymatic modification of the antigenic compounds upon immediate and delayed reactions. Infect Immun. 1975 Jun;11(6):1187–1194. doi: 10.1128/iai.11.6.1187-1194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunayama H. Studies on the antigenic activities of yeasts. IV. Analysis of the antigenic determinant groups of the mannan of Candida albicans serotype A. Jpn J Microbiol. 1970 Jan;14(1):27–39. doi: 10.1111/j.1348-0421.1970.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sunayama H., Saito T. Studies on the antigenic activity of yeasts. I. Analysis of the determinant groups of the mannan of Saccharomyces cerevisiae. Jpn J Microbiol. 1968 Mar;12(1):19–24. doi: 10.1111/j.1348-0421.1968.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sunayama H. Studies on the antigenic activities of yeasts. 3. Isolation and inhibition assay of the oligosaccharides from acid-hydrolysate of mannan of Candida albicans. Jpn J Microbiol. 1969 Mar;13(1):95–101. doi: 10.1111/j.1348-0421.1969.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sunayama H. Studies on the antigenic activities of yeasts. II. Isolation and inhibition assay of the oligosaccharides from acetolysate of the mannan of Candida albicans. Jpn J Microbiol. 1968 Dec;12(4):413–422. doi: 10.1111/j.1348-0421.1968.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Suzuki M., Sakaguchi O. Biochemical and immunochemical studies on fungi. VII. Isolation of a galactomannan produced in the culture of Trichophytou rubrum. Chem Pharm Bull (Tokyo) 1967 Jul;15(7):976–979. doi: 10.1248/cpb.15.976. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Takeda N. Serologic cross-reactivity of the D-galacto-D-mannans isolated from several pathogenic fungi against anti-Hormodendrum pedrosoi serum. Carbohydr Res. 1975 Mar;40(1):193–197. doi: 10.1016/s0008-6215(00)82681-6. [DOI] [PubMed] [Google Scholar]
- Suzuta T. [Experimental method for allergy (3)]. Tanpakushitsu Kakusan Koso. 1969 Aug;14(9):823–828. [PubMed] [Google Scholar]
- Takeda N., Suzuki S. [Immunochemical studies on chromoblastomycosis. 1. Isolation and purification of antigenic polysaccharides from mycelia and culture filtrates of Hormodendrum pedrosoi, H. compactum and H. determatitidis]. Nihon Saikingaku Zasshi. 1974 Mar;29(2):369–378. [PubMed] [Google Scholar]
- Takeda N., Suzuki S. [Immunochemical studies on chromoblastomycosis. II. Serological relationship among galactomannans from 3 species of the genus Hormodendrum, H. pedrosio, H. compactum and H. dermatitidis]. Nihon Saikingaku Zasshi. 1974 Sep;29(5):757–763. [PubMed] [Google Scholar]




