Abstract
Ocular neovascularization can affect almost all the tissues of the eye: the cornea, the iris, the retina, and the choroid. Pathological neovascularization is the underlying cause of vision loss in common ocular conditions such as diabetic retinopathy, retinopathy of prematurity and age-related macular neovascularization. Glycosylation is the most common covalent posttranslational modification of proteins in mammalian cells. A growing body of evidence demonstrates that glycosylation influences the process of angiogenesis and impacts activation, proliferation, and migration of endothelial cells as well as the interaction of angiogenic endothelial cells with other cell types necessary to form blood vessels. Recent studies have provided evidence that members of the galectin class of β-galactoside-binding proteins modulate angiogenesis by novel carbohydrate-based recognition systems involving interactions between glycans of angiogenic cell surface receptors and galectins. This review discusses the significance of glycosylation and the role of galectins in the pathogenesis of ocular neovascularization.
Keywords: angiogenesis, choroidal neovascularization, corneal neovascularization, galectin-3, glycans, glycosylation, integrins, neovascularization, retinal neovascularization
Introduction
Ocular angiogenesis is a major cause of blindness and visual impairment. Angiogenesis or neovascularization affects almost all tissues of the eye: the cornea, the iris, the retina, and the choroid (Adamis et al. 1999). Epiretinal neovascularization in patients with proliferative diabetic retinopathy and choroidal neovascularization (CNV) in patients with neovascular age-related macular degeneration (AMD) are the two most common causes of catastrophic vision loss. Much like tumor-induced vessels, the ocular angiogenic vessels are leaky and lack structural integrity (Dorrell et al. 2007). The resultant hemorrhage and fibrosis cause severe damage to the ocular tissues and frequently leads to vision loss or impairment (Dorrell et al. 2007).
Angiogenesis, the formation of new blood vessels from preexisting vasculature, is a tightly regulated process that begins when the endothelial cells of a mature blood vessel wall are activated by angiogenic factors that include, but are not limited to, the vascular endothelial cell growth factor (VEGF) and basic fibroblast growth factor (bFGF) families of cytokines (Cross and Claesson-Welsh 2001). Activation promotes the loosening of endothelial cells from their basement membrane and the supporting periendothelial cells, thereby allowing them to migrate, proliferate, and ultimately form a capillary lumen, which is stabilized by pericytes and smooth muscle cells. Glycosylation, the most common covalent posttranslational modification of proteins, profoundly influences the process of angiogenesis. A growing body of evidence demonstrates that glycosylation can impact activation, proliferation, and migration of endothelial cells as well as the interaction of angiogenic endothelial cells with other cell types necessary to form blood vessels. Early studies have shown that N-linked glycosylation is critical for angiogenesis, as inhibition of enzymes early in the glycosylation pathway block vessel growth. The inhibition of N acetylglucosamine-1-phosphotransferase, which catalyzes the first step of glycoprotein biosynthesis, blocks endothelial cell proliferation and alters endothelial cell–extracellular matrix (ECM) interactions (Tiganis et al. 1992). Similarly, inhibition of glucosidase I and glucosidase II, which sequentially remove terminal glucose residues from the N-acetylglucosamine-1-phosphotransferase product by castranospermidine and N-methyl-1-deoxynojirimycin, reduce endothelial cell migration in vitro and FGF-induced angiogenesis in vivo (Pili et al. 1995). Likewise, inhibition by 1-deoxymannojiriniycin of Golgi-α-mannosidase, which acts on the glucosidase I/II product, blocks angiogenesis in vitro (Nguyen et al. 1992). Treatment with swainsonine, an inhibitor of Golgi α-mannosidase II, also markedly reduces vessel density and distorts the placental angiogenesis in vivo (Hafez et al. 2007). O-Linked N-acetylglucosamine (O-GlcNAc) modifications also play a role in angiogenesis. Recent studies have shown that increased expression of O-GlcNAc transferase, which catalyzes the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine (UDP-GlcNAc) to serine and threonine, enhances the angiogenic potential of prostate cancer cells in part by modulating the function of FOXM1 (Lynch et al. 2012). However, in a different study, increased expression of O-GlcNAc glycans was shown to reduce vascular sprouting from aortic rings, as well as migration and capillary tubule formation of endothelial cells (Luo et al. 2008). Accordingly, removal of O-GlcNAc residues, by overexpression of O-GlcNAcase, enhanced angiogenesis (Luo et al. 2008).
Angiogenesis is predominantly mediated by a family of VEGF receptors (Hoeben et al. 2004; Breen 2007; Otrock et al. 2007; Roskoski 2008) and integrins (Garmy-Susini and Varner 2008; Silva et al. 2008; Contois et al. 2009). Like most cell surface proteins, VEGF and integrin receptors are glycosylated, although their role in angiogenesis with respect to their glycosylation pattern is only beginning to be characterized. Recent studies have provided evidence that members of the galectin class of β-galactoside-binding proteins also have the potential to modulate angiogenesis by novel carbohydrate-based recognition systems involving interactions between glycans of angiogenic cell surface receptors and galectins (Nangia-Makker et al. 2000; Thijssen et al. 2006, 2007; Hsieh et al. 2008; Markowska et al. 2010; Delgado et al. 2011; Croci et al. 2014). With respect to ocular angiogenesis, galectin-3 (Gal-3) has been shown to promote corneal neovascularization (Markowska et al. 2010, 2011).
Gal-3 and angiogenesis
Gal-3 is a member of the galectin family of mammalian lectins characterized by a conserved sequence within the carbohydrate recognition domain (CRD) that has affinity for β-galactoside structures. Extracellularly, the lectin is assumed to mediate cell–cell and cell–matrix interactions by binding to lactosamine-containing cell surface glycoconjugates via the CRD. That Gal-3 is a novel proangiogenic molecule was first suggested by Nangia-Makker et al. (2000) who reported that tumor angiogenesis induced by subcutaneous injections of breast carcinoma cells in an animal model is significantly greater when the carcinoma cells express Gal-3 as compared with Gal-3-null controls, and that exogenous Gal-3 promotes endothelial cell migration and capillary tubule formation in vitro. In addition, it was reported that modified citrus pectin, a galactose-rich polysaccharide that binds to Gal-3, and possibly also to other members of the galectin family, reduces bFGF-mediated migration of endothelial cells, suggesting that one or more members of the galectin family may participate in bFGF-mediated angiogenesis (Nangia-Makker et al. 2002). More recent studies in our laboratory aimed at characterization of the mechanism by which Gal-3 promotes angiogenesis revealed that Gal-3 is a mediator of VEGF- and bFGF-mediated angiogenic response (Markowska et al. 2010). In these studies, we demonstrated that Gal-3 inhibitors, β-lactose and dominant negative Gal-3, reduce VEGF- and bFGF-mediated angiogenesis in vitro, and that VEGF- and bFGF-mediated angiogenic response is reduced in Gal-3 knockdown cells and Gal-3−/− animals (Markowska et al. 2010). A well-known proangiogenic integrin, αvβ3, was identified as a Gal-3-binding protein. Anti-αvβ3 integrin function-blocking antibodies significantly inhibited the Gal-3-induced angiogenesis in vitro. Furthermore, Gal-3 promoted the clustering of integrin αvβ3 and activated focal adhesion kinase (FAK). The knockdown of GnTV, an enzyme that synthesizes high-affinity glycan ligands for Gal-3, reduced: (i) complex N-glycans on αvβ3 integrins and (ii) VEGF- and bFGF-mediated angiogenesis. Taken together, these data suggest that Gal-3 modulates VEGF- and bFGF-mediated angiogenesis, at least in part, by binding via its CRD to the GnTV synthesized N-glycans of integrin αvβ3, and subsequently activating the signaling pathways that promote the growth of new blood vessels. Additional studies in our laboratory demonstrated that Gal-3 also modulates cell surface expression and activation of VEGF-R2 in human endothelial cells (Markowska et al. 2011). In this study, we found that Gal-3 interacts with VEGF-R2 in a carbohydrate-dependent manner, Gal-3 promotes VEGF-R2 phosphorylation in time- and dose-dependent manner, VEGF-R2 bears GnTV-modified N-glycans, and knockdown of GnTV or Gal-3 reduces the cell surface expression of VEGF-R2 (Markowska et al. 2011). These data led us to propose that Gal-3 oligomers cross-link VEGF receptors into a lattice formation on the cell surface and thereby delay their removal by endocytosis and enhance VEGF-R2 signaling and angiogenesis (Markowska et al. 2011).
Gal-3 may also modulate angiogenesis by inducing the expression of MMPs. In a recent study, Argueso and colleagues (Mauris et al. 2014) have demonstrated that Gal-3 plays a key role in destabilizing cell–cell interactions by interacting with and clustering CD147 on the epithelial cell surface. In this study, the authors identified CD147 as a membrane receptor for Gal-3 in human keratinocytes and demonstrated that Gal-3 initiates keratinocyte cell–cell disassembly by inducing MMP expression in a CD147-dependent manner. These findings are relevant to angiogenesis because endothelial cells express CD147 and disruption of cell–cell assembly and the degradation of the ECM to mitigate the physical constraint to cell movement is the first step in the onset of angiogenesis. Interestingly, MMPs have been shown to cleave Gal-3 to release a highly proangiogenic fragment that shows diminished self-association and ability to hemagglutinate red blood cells, but drastically improved: (i) binding affinity to laminin and endothelial cells, (ii) chemotactic properties toward endothelial cells, (iii) ability to upregulate pFAK in migrating endothelial cells and promote angiogenesis (Nangia-Makker et al. 2010). Thus, it is reasonable to speculate that Gal-3 may also modulate angiogenesis by inducing the expression of MMPs which, in turn, cleave Gal-3 itself to promote angiogenesis. Studies of tumor-associated macrophages have shown that Gal-3 also promotes angiogenesis by accelerating M2 macrophage infiltration into tumors (Jia et al. 2013) and by enhancing the VEGF secretion from macrophages (Machado et al. 2014).
Glycobiology of ocular angiogenesis
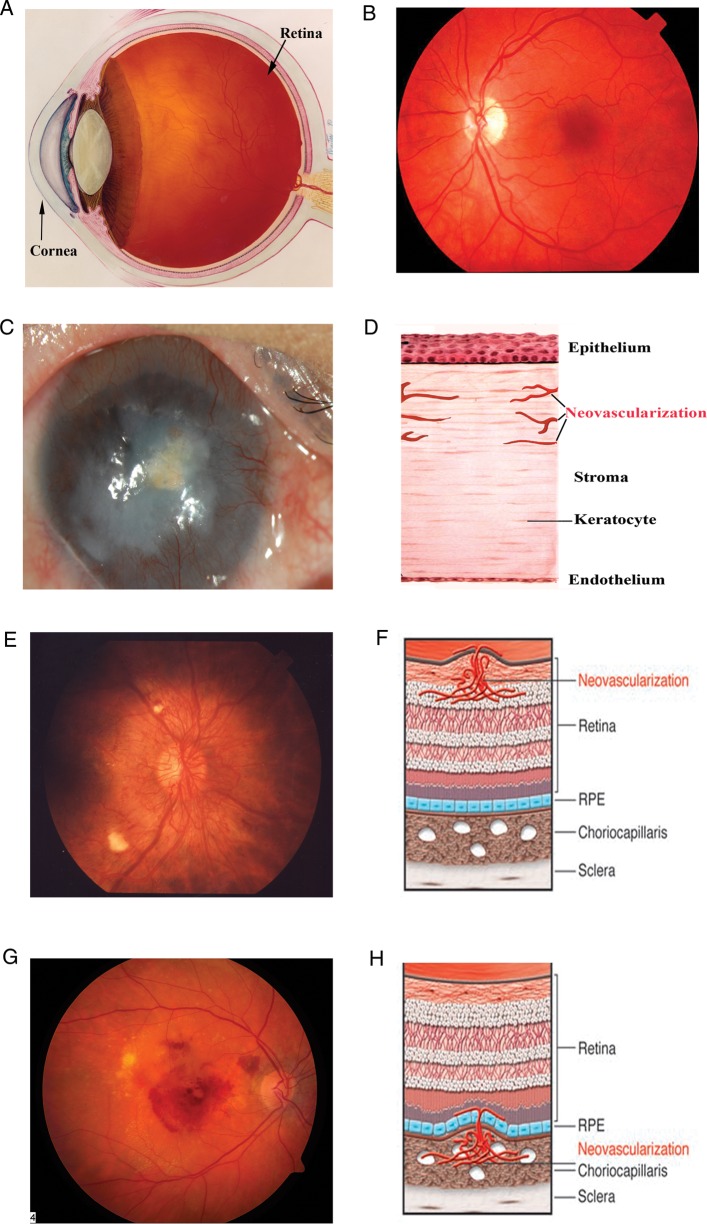
Vision requires that ocular tissues remain transparent such that light is able to reach photoreceptors undistorted. Complex mechanisms are in place to ensure transparency and provide a route for cells to obtain metabolites and oxygen (Figure 1A and B). Resourceful arrangement of the vasculature and partial avascularity combine to complete this task. As described earlier, ocular neovascularization nearly always impairs vision. Corneal, retinal and CNV are among serious clinical conditions encountered in tertiary-care ophthalmology clinics around the world.
Fig. 1.
Schematic and photographic representation of the eye and corneal, retinal, and CNV. (A) Schematic depiction of the eye. (B) The fundus, i.e., the inner lining of a normal eye. (C and D) Normal cornea is transparent and avascular; in response to trauma, graft rejection or infection, blood vessels from the limbus (region where transparent cornea meets the opaque sclera) invade the cornea. (E and F) The retina is a highly ordered, multilayered structure that is richly vascularized. Diabetic retinopathy can lead to ischemia and neovascularization on the surface of the retina. (G and H) AMD can be associated with subretinal neovascularization originating from the choriocapillaris, and this can lead to subretinal hemorrhage. Credit: (A), (E) and (G) downloaded from National Eye Institute, NIH website with permission (ref.: NEA04, EDA01, EDA 24); (B) provided by J. S. Duker; (C) provided by Sugaya Satoshi and Tohru Sakimoto; (F) and (H) from Friedlander (2007) with permission.
Corneal neovascularization
Corneal neovascularization (Figure 1C and D) is a vision-threatening condition affecting ∼1.4 million individuals each year in the United States alone (Chang et al. 2001; Shakiba et al. 2009). It is associated with a wide range of ocular disorders including viral, bacterial and parasitic infections, inflammatory disorders of the ocular surface and trauma. The complications of corneal neovascularization include corneal scarring, edema, lipidic deposition and increased risk of graft rejection. The frequency of rejection of corneal grafts placed in vascularized high-risk host beds can reach as high as 90%. This is in sharp contrast to the >90% acceptance rate of corneal grafts placed in avascular host beds. Thus, the development of effective strategies to prevent the growth of blood vessels in the cornea is a high priority, not only to prevent corneal graft rejection but also to treat numerous other inflammatory disorders of the ocular surface.
Increased expression of VEGF is a common trigger for loss of corneal angiogenic privilege. Vascularized corneas express significantly greater amounts of VEGF and its receptors compared with normal corneas (Philipp et al. 2000). VEGF is secreted by the corneal epithelial cells and corneal fibroblasts upon injury and by macrophages subsequently recruited to the site (Cursiefen et al. 2004; Nakao et al. 2007; Sivak et al. 2011). Activation of conjunctival blood vessels by interaction of VEGF with VEGF-R2 promotes vessel growth (Mimura et al. 2001). Multiple other factors such as bFGF (Knighton et al. 1990; Gaudric et al. 1992), angiopoeitins (Asahara et al. 1998) and PDGF (Cao et al. 2002) are also involved in corneal neovascularization. Integrins also play a critical role in corneal neovascularization. Integrins αvβ5 and α5β1 are preferentially expressed on the neovasculature in the corneal alkaline burn model (Zhang et al. 2002). Moreover, inhibition of integrin α5β1 by a small molecule inhibitor, JSM5562, significantly reduces corneal neovascularization in a mouse scrape model (Muether et al. 2007). Interestingly, one study has shown that while αv integrin antagonist inhibits FGF pellet-induced corneal neovascularization, it did not inhibit inflammatory corneal angiogenesis induced by chemical burns (Klotz et al. 2000). The authors concluded that angiogenic pathways independent of αv integrin are involved in corneal inflammatory angiogenesis. In contrast, retinal (Luna et al. 1996; Riecke et al. 2001; Economopoulou et al. 2005; Yoshida et al. 2012), choroidal (Honda et al. 2009) and tumor angiogenesis is inhibited by αv integrin antagonists. Thus, it appears that there are context- and tissue-dependent differences in angiogenic pathways.
Normally avascular cornea has been extensively used as the in vivo model to investigate the molecular mechanism of angiogenesis and to examine the efficacy of the inhibitors and activators of the growth of new blood vessels. In these assays, known as corneal micropocket assays, standardized slow-release pellets containing test substances are implanted into the corneal stroma. The vessel area representing the extent of angiogenesis is calculated 5–8 days after pellets are implanted in the corneas (Rogers et al. 2007). Using mouse corneal micropocket assays, we have shown that Gal-3 directly promotes corneal angiogenesis in vivo. In this study, the vessel area representing the extent of angiogenesis was calculated 5 days after pellets containing various concentrations of Gal-3 were implanted in the mouse corneas. In the concentration range tested (20–160 ng Gal-3/pellet), the extent of angiogenesis increased in a dose-dependent manner (Markowska et al. 2010). A dominant negative inhibitor of Gal-3 that competes with the CRD, but is unable to oligomerize, effectively inhibited angiogenesis advanced by full-length Gal-3, suggesting that Gal-3 promotes angiogenesis in the cornea in a carbohydrate-dependent manner (Markowska et al. 2010).
Alterations in the N-glycosylation pathway markedly influence the progression of corneal neovascularization. VEGF-A-induced as well as suture-induced inflammatory corneal neovascularization is significantly reduced in the knockout mice deficient in GnTV and Gal-3 (Markowska et al. 2010, 2011). GnTV synthesizes N-glycan intermediates, the β1, 6GlcNAc-branched glycans, that are elongated with N-acetyllactosamines to create high-affinity ligands for Gal-3 (Dennis et al. 2002). Its disruption prevents interaction of Gal-3 with N-glycan moieties of angiogenic cell surface receptors VEGF-R2 and αvβ3 integrins and, thereby, results in reduced neovascularization (Markowska et al. 2010, 2011). Gal-3 also mediates the interaction of integrin α3β1 and NG2 proteoglycan to promote cell–cell communication between pericytes and endothelial cells during the early stages of corneal angiogenesis (Fukushi et al. 2004). In the corneal micropocket assays, Gal-1 and Gal-8 also promote angiogenesis (Markowska and Panjwani unpublished). Currently, we are characterizing the mechanism by which these galectins promote corneal angiogenesis.
In conclusion, carbohydrate-mediated recognition plays a critical role in corneal neovascularization. In contrast, much less is known about the glycobiology of retinal and CNV.
Retinal and choroidal neovascularization
Epiretinal neovascularization (Figure 1E and F) is characteristic of proliferative diabetic retinopathy, retinopathy of prematurity and retinal vein occlusion. Proliferative diabetic retinopathy is a leading cause of blindness in working age population affecting ∼20.8 million people worldwide (Afzal et al. 2007; Morello 2007). Oxidative injury from diabetic hyperglycemia and the resulting hypoxia activates retinal vasculature in diabetic retinopathy (Qazi et al. 2009). Activated retinal vessels grow into the vitreous causing hemorrhage. This leads to degeneration and eventual collapse of the vitreous that pulls the retina and results in retinal detachment, and consequently impairment of vision. VEGF is the primary mediator of retinal neovascularization. Vitreous VEGF levels are significantly greater in patients with diabetic retinopathy than controls (Adamis et al. 1994). Moreover, the severity of retinopathy is closely associated with VEGF expression (Funatsu et al. 2002). Expression of VEGF receptors is also increased in retinal neovasculature. Cadavers with a history of diabetes mellitus have higher levels of vitreal VEGF-R1 and VEGF-R2 than controls. Integrins also play a key role in retinal neovascularization. Increased expression of integrin αvβ3 and αvβ5 was observed in tissues from patients with diabetic retinopathy (Friedlander et al. 1996; Ljubimov et al. 1998). Inhibition of integrin α4 reduced expression of VEGF, as well as vascular hemorrhage in vivo (Iliaki et al. 2009). Moreover, inhibitors of various integrins including αvβ3, αvβ5 and α5β1 have been shown to inhibit retinal neovascularization in animal models of proliferative retinopathy (Riecke et al. 2001; Iliaki et al. 2009; Yoshida et al. 2012) and retinopathy of prematurity (Luna et al. 1996; Witmer et al. 2002; Economopoulou et al. 2005; Wilkinson-Berka et al. 2006). Other factors thought to play a role in retinal neovascularization include stromal-derived growth factor 1 and its receptor CXCR4 (Lima e Silva et al. 2007), platelet-derived growth factor B, placental growth factor and pigment epithelium-derived factor (Seo et al. 2000; Luttun et al. 2002; Mori et al. 2002; Ogata et al. 2002, 2007).
CNV, the growth of abnormal blood vessels underneath the retina (Figure 1G and H), is the major cause of severe vision loss in patients with neovascular AMD. CNV affects nearly 11.1 million people in the United States alone and is the leading cause of irreversible blindness among those over 65 in the developed world (Klein et al. 1995). Pathological choroidal neovasculature originates from the choroid. The resulting vessels extend through the Bruch's membrane and retinal pigment epithelium (RPE) causing detachment of the photoreceptors from the RPE. VEGF is also a key mediator of CNV. VEGF expression is increased in the neovascular membranes with AMD (Frank et al. 1996; Kvanta et al. 1996; Lopez et al. 1996; Kliffen et al. 1997; Hera et al. 2005) and in AMD vitreous (Aiello et al. 1994; Wells et al. 1996; Tong et al. 2006). In animal models, overexpression of VEGF promotes CNV (Spilsbury et al. 2000; Csaky et al. 2004) and its inhibition blocks neovascularization (Krzystolik et al. 2002; Saishin et al. 2003; Jo et al. 2014). Expression of proangiogenic integrin avb3 is also increased in AMD neovasculature (Friedlander et al. 1996). Furthermore, in a laser model of CNV, inhibition of integrin αvβ3 significantly reduces the extent of neovascularization (Honda et al. 2009). Integrin α5β1 is also expressed on choroidal neovasculature and treatment with integrin α5β1 small molecule inhibitor, JSM6427, is able to prevent and regress CNV in the mouse model (Umeda et al. 2006).
Very few studies have been reported on glycobiology of retinal or CNV. It has been demonstrated that Gal-3, by serving as a receptor for advanced glycation end products (AGEs), modulates retinal angiogenesis in diabetes (Stitt et al. 2005). Specifically, in the mouse model of diabetic retinopathy, prevention of AGE formation or deletion of Gal-3 prevented acute diabetic retinopathy (Canning et al. 2007). Increased O-GlcNAc modifications in neovascular retinas strongly correlates with reduced migration of pericytes observed in diabetic vasculature (Gurel et al. 2013) but its precise role in the regulation of retinal neovascularization has not been evaluated.
Conclusions
Ocular neovascularization is a leading cause of vision loss. Carbohydrate recognition is a largely underappreciated regulatory mechanism in the pathogenesis of ocular angiogenesis that warrants investigation in ocular disease as it may provide valuable mechanistic insight as well as potential therapeutic targets. Considering that Gal-3 is a mediator of VEGF-mediated angiogenic response and VEGF is a primary mediator of retinal as well as CNV, it is very likely that carbohydrate-mediated recognition plays a prominent role in the mechanisms modulating the growth of abnormal blood vessels in the retina and choroid. It is our hope that this review will provide impetus for future studies to characterize the role of carbohydrate-based, galectin-mediated angiogenic pathways in the pathogenesis of retinal and CNV. Also, future studies targeting galectins to develop novel therapeutic strategies to control ocular angiogenesis are likely to prove rewarding. Therapeutic strategies to prevent abnormal angiogenesis have, thus far, largely targeted VEGF since it plays a central role in the pathogenesis of ocular neovascularization. A major limitation of VEGF targeting therapies is the adverse effect of sustained VEGF inhibition on the choriocapillaris. Long-term inhibition of VEGF leads to chorioretinal atrophy (Yamazaki et al. 2012; Rofagha et al. 2013; Fernandez-Robredo et al. 2014). Intravitreal injections of bevacizumab (Genentech), a full-length recombinant humanized antibody that binds to all isoforms of VEGF, have been shown to cause a significant reduction of choriocapillaris endothelial cell fenestrations in primate eyes (Peters et al. 2007; Schraermeyer and Julien 2012). Despite advances in anti-VEGF therapies designed to combat choroidal and retinal neovascularization, many patients do not significantly improve (Mitchell 2011; Patel et al. 2011). Why some patients with CNV and proliferative diabetic retinopathy do not respond to anti-VEGF therapy is a very important and a clinically relevant question. In this respect, Croci et al. have identified a glycosylation-dependent pathway that supports angiogenesis in a VEGF-independent manner (Croci et al. 2014). This study revealed that anti-VEGF refractory tumors exhibit a distinct glycosylation signature that interferes with Gal-1-induced angiogenesis Specifically, it was demonstrated that vessels within anti-VEGF-sensitive tumors exhibit high levels of alpha2-6-linked sialic acid, which prevent Gal-1–endothelial cell interactions. In contrast, vessels with the anti-VEGF refractory tumors display glycosylation patterns that facilitate Gal-1–endothelial cell interactions. It was further demonstrated that silencing of Gal-1 itself or a glycosyltransferase that synthesizes Gal-1 ligands in endothelial cells converted refractory tumors into anti-VEGF-sensitive tumors. These findings provide impetus to investigate whether vitreous and retina of patients who do not respond to anti-VEGF therapy have distinct glycosylation signature compared with those who respond. Unfortunately, such samples are not readily available for analyses. Regardless, it is logical to assume that galectin-based strategies hold the potential to develop drugs to enhance the efficacy of anti-VEGF treatment. Last but not least, current anti-VEGF-based therapies are associated with a high rate of retinal fibrosis and geographic atrophy (Kuiper et al. 2008; Van Geest et al. 2012). Therefore, there is a major unmet need for developing dual target drugs for inhibition of both angiogenesis and fibrosis. In this respect targeting galectins, particularly Gal-3 may prove to be beneficial. As described above in the section on Gal-3 and angiogenesis, Gal-3 is an important mediator of VEGF-mediated angiogenic response. These findings in conjunction with reports showing that Gal-3 is also a profibrotic protein that modulates TGF-β-driven fibrosis (Henderson et al. 2006, 2008, 2012; MacKinnon et al. 2008, 2012) suggest that inhibiting carbohydrate-mediated Gal-3 function is likely to inhibit both angiogenesis and fibrosis.
Funding
The work carried out in the author's laboratory was supported by National Institutes of Health Grants EY009349 and EY007088, the Massachusetts Lions Eye Research Fund, the New England Corne al Transplant Fund and an unrestricted award from Research to Prevent Blindness.
Conflict of interest statement
None declared.
Abbreviations
AGEs, advanced glycation end products; AMD, age-related macular degeneration; bFGF, basic fibroblast growth factor; CNV, choroidal neovascularization; CRD, carbohydrate recognition domain; ECM, extracellular matrix; FAK, focal adhesion kinase; Gal-3, galectin-3; O-GlcNAc, O-linked N-acetylglucosamine; RPE, retinal pigment epithelium; UDP-GlcNAc, UDP-N-acetylglucosamine; VEGF, vascular endothelial cell growth factor.
References
- Adamis AP, Aiello LP, D'Amato RA. Angiogenesis and ophthalmic disease. Angiogenesis. 1999;3:9–14. doi: 10.1023/a:1009071601454. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: Therapeutic opportunities. Microvasc Res. 2007;74:131–144. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Breen EC. VEGF in biological control. J Cell Biochem. 2007;102:1358–1367. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- Canning P, Glenn JV, Hsu DK, Liu FT, Gardiner TA, Stitt AW. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp Diabetes Res. 2007;2007:51837. doi: 10.1155/2007/51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Contois L, Akalu A, Brooks PC. Integrins as “functional hubs” in the regulation of pathological angiogenesis. Semin Cancer Biol. 2009;19:318–328. doi: 10.1016/j.semcancer.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- Csaky KG, Baffi JZ, Byrnes GA, Wolfe JD, Hilmer SC, Flippin J, Cousins SW. Recruitment of marrow-derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res. 2004;78:1107–1116. doi: 10.1016/j.exer.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado VM, Nugnes LG, Colombo LL, Troncoso MF, Fernandez MM, Malchiodi EL, Frahm I, Croci DO, Compagno D, Rabinovich GA, et al. Modulation of endothelial cell migration and angiogenesis: A novel function for the “tandem-repeat” lectin galectin-8. FASEB J. 2011;25:242–254. doi: 10.1096/fj.09-144907. [DOI] [PubMed] [Google Scholar]
- Dennis K, Uittenbogaard M, Chiaramello A, Moody SA. Cloning and characterization of the 5′-flanking region of the rat neuron-specific Class III beta-tubulin gene. Gene. 2002;294:269–277. doi: 10.1016/s0378-1119(02)00801-6. [DOI] [PubMed] [Google Scholar]
- Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: Basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52:3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Economopoulou M, Bdeir K, Cines DB, Fogt F, Bdeir Y, Lubkowski J, Lu W, Preissner KT, Hammes HP, Chavakis T. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood. 2005;106:3831–3838. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Robredo P, Sancho A, Johnen S, Recalde S, Gama N, Thumann G, Groll J, Garcia-Layana A. Current treatment limitations in age-related macular degeneration and future approaches based on cell therapy and tissue engineering. J Ophthalmol. 2014;2014:510285. doi: 10.1155/2014/510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117:576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Varner JA. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res Biol. 2008;6:155–163. doi: 10.1089/lrb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudric A, N'Guyen T, Moenner M, Glacet-Bernard A, Barritault D. Quantification of angiogenesis due to basic fibroblast growth factor in a modified rabbit corneal model. Ophthalmic Res. 1992;24:181–188. doi: 10.1159/000267166. [DOI] [PubMed] [Google Scholar]
- Gurel Z, Sieg KM, Shallow KD, Sorenson CM, Sheibani N. Retinal O-linked N-acetylglucosamine protein modifications: Implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol Vis. 2013;19:1047–1059. [PMC free article] [PubMed] [Google Scholar]
- Hafez SA, Caceci T, Freeman LE, Panter KE. Angiogenesis in the caprine caruncles in non-pregnant and pregnant normal and swainsonine-treated does. Anat Rec (Hoboken) 2007;290:761–769. doi: 10.1002/ar.20548. [DOI] [PubMed] [Google Scholar]
- Henderson NC, MacKinnon AC, Rooney C, Sethi T. Galectin-3: A central regulator of chronic inflammation and tissue fibrosis. In: Klyosov AA, Traber PG, editors. Galectins and Disease Implications for Targeted Therapeutics. Washington: American Chemical Society; 2012. pp. 377–390. [Google Scholar]
- Henderson NC, MacKinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, MacKinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hera R, Keramidas M, Peoc'h M, Mouillon M, Romanet JP, Feige JJ. Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am J Ophthalmol. 2005;139:589–596. doi: 10.1016/j.ajo.2004.11.064. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Honda S, Nagai T, Negi A. Anti-angiogenic effects of non-peptide integrin alphavbeta3 specific antagonist on laser-induced choroidal neovascularization in mice. Graefes Arch Clin Exp Ophthalmol. 2009;247:515–522. doi: 10.1007/s00417-008-1010-5. [DOI] [PubMed] [Google Scholar]
- Hsieh SH, Ying NW, Wu MH, Chiang WF, Hsu CL, Wong TY, Jin YT, Hong TM, Chen YL. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008;27:3746–3753. doi: 10.1038/sj.onc.1211029. [DOI] [PubMed] [Google Scholar]
- Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:4898–4904. doi: 10.1167/iovs.08-2013. [DOI] [PubMed] [Google Scholar]
- Jia W, Kidoya H, Yamakawa D, Naito H, Takakura N. Galectin-3 accelerates M2 macrophage infiltration and angiogenesis in tumors. Am J Pathol. 2013;182:1821–1831. doi: 10.1016/j.ajpath.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Jo DH, Kim S, Kim D, Kim JH, Jon S. VEGF-binding aptides and the inhibition of choroidal and retinal neovascularization. Biomaterials. 2014;35:3052–3059. doi: 10.1016/j.biomaterials.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- Kliffen M, van der Schaft TL, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech. 1997;36:106–122. doi: 10.1002/(SICI)1097-0029(19970115)36:2<106::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Klotz O, Park JK, Pleyer U, Hartmann C, Baatz H. Inhibition of corneal neovascularization by alpha(v)-integrin antagonists in the rat. Graefes Arch Clin Exp Ophthalmol. 2000;238:88–93. doi: 10.1007/s004170050015. [DOI] [PubMed] [Google Scholar]
- Knighton DR, Phillips GD, Fiegel VD. Wound healing angiogenesis: Indirect stimulation by basic fibroblast growth factor. J Trauma. 1990;30:S134–S144. [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS ONE. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara MC, Lauer T, Aslam S, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- Luna J, Tobe T, Mousa SA, Reilly TM, Campochiaro PA. Antagonists of integrin alpha v beta 3 inhibit retinal neovascularization in a murine model. Lab Invest. 1996;75:563–573. [PubMed] [Google Scholar]
- Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:651–657. doi: 10.1161/ATVBAHA.107.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CM, Andrade LN, Teixeira VR, Costa FF, Melo CM, dos Santos SN, Nonogaki S, Liu FT, Bernardes ES, Camargo AA, et al. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFbeta1-induced macrophages. Cancer Med. 2014;3:201–214. doi: 10.1002/cam4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- MacKinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207:1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J Cell Sci. 2014;127:3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Amano S, Usui T, Kaji Y, Oshika T, Ishii Y. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp Eye Res. 2001;72:71–78. doi: 10.1006/exer.2000.0925. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: Comparison of ranibizumab and bevacizumab. Curr Med Res Opin. 2011;27:1465–1475. doi: 10.1185/03007995.2011.585394. [DOI] [PubMed] [Google Scholar]
- Morello CM. Etiology and natural history of diabetic retinopathy: An overview. Am J Health Syst Pharm. 2007;64:S3–S7. doi: 10.2146/ajhp070330. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, McVey D, Wei L, Campochiaro PA. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43:2428–2434. [PubMed] [Google Scholar]
- Muether PS, Dell S, Kociok N, Zahn G, Stragies R, Vossmeyer D, Joussen AM. The role of integrin alpha5beta1 in the regulation of corneal neovascularization. Exp Eye Res. 2007;85:356–365. doi: 10.1016/j.exer.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, et al. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: Role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171:1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, Raz A. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Folkman J, Bischoff J. 1-Deoxymannojirimycin inhibits capillary tube formation in vitro. Analysis of N-linked oligosaccharides in bovine capillary endothelial cells. J Biol Chem. 1992;267:26157–26165. [PubMed] [Google Scholar]
- Ogata N, Matsuoka M, Matsuyama K, Shima C, Tajika A, Nishiyama T, Wada M, Jo N, Higuchi A, Minamino K, et al. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab. 2007;92:1176–1179. doi: 10.1210/jc.2006-2249. [DOI] [PubMed] [Google Scholar]
- Ogata N, Wada M, Otsuji T, Jo N, Tombran-Tink J, Matsumura M. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1168–1175. [PubMed] [Google Scholar]
- Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Patel RD, Momi RS, Hariprasad SM. Review of ranibizumab trials for neovascular age-related macular degeneration. Semin Ophthalmol. 2011;26:372–379. doi: 10.3109/08820538.2011.570845. [DOI] [PubMed] [Google Scholar]
- Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, Schraermeyer U. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- Pili R, Chang J, Partis RA, Mueller RA, Chrest FJ, Passaniti A. The alpha-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res. 1995;55:2920–2926. [PubMed] [Google Scholar]
- Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecke B, Chavakis E, Bretzel RG, Linn T, Preissner KT, Brownlee M, Hammes HP. Topical application of integrin antagonists inhibits proliferative retinopathy. Horm Metab Res. 2001;33:307–311. doi: 10.1055/s-2001-15279. [DOI] [PubMed] [Google Scholar]
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Silva RL, Callahan K, Schoch C, Ahlheim M, Lai H, Kane F, Brazzell RK, Bodmer D, Campochiaro PA. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest Ophthalmol Vis Sci. 2003;44:4989–4993. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Julien S. Formation of immune complexes and thrombotic microangiopathy after intravitreal injection of bevacizumab in the primate eye. Graefes Arch Clin Exp Ophthalmol. 2012;250:1303–1313. doi: 10.1007/s00417-012-2055-z. [DOI] [PubMed] [Google Scholar]
- Seo MS, Okamoto N, Vinores MA, Vinores SA, Hackett SF, Yamada H, Yamada E, Derevjanik NL, LaRochelle W, Zack DJ, et al. Photoreceptor-specific expression of platelet-derived growth factor-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiba Y, Mansouri K, Arshadi D, Rezaei N. Corneal neovascularization: Molecular events and therapeutic options. Recent Pat Inflamm Allergy Drug Discov. 2009;3:221–231. doi: 10.2174/187221309789257450. [DOI] [PubMed] [Google Scholar]
- Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: The keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Ostriker AC, Woolfenden A, Demirs J, Cepeda R, Long D, Anderson K, Jaffee B. Pharmacologic uncoupling of angiogenesis and inflammation during initiation of pathological corneal neovascularization. J Biol Chem. 2011;286:44965–44975. doi: 10.1074/jbc.M111.294967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA. Impaired retinal angiogenesis in diabetes: Role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: Opportunities for combined cancer therapy. Blood. 2007;110:2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T, Leaver DD, Ham K, Friedhuber A, Stewart P, Dziadek M. Functional and morphological changes induced by tunicamycin in dividing and confluent endothelial cells. Exp Cell Res. 1992;198:191–200. doi: 10.1016/0014-4827(92)90371-e. [DOI] [PubMed] [Google Scholar]
- Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Umeda N, Kachi S, Akiyama H, Zahn G, Vossmeyer D, Stragies R, Campochiaro PA. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, Murthy R, Chibber R, Nunn A, Molinatti PA, Kohner EM, Gregor ZJ. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996;80:363–366. doi: 10.1136/bjo.80.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Jones D, Taylor G, Jaworski K, Kelly DJ, Ludbrook SB, Willette RN, Kumar S, Gilbert RE. SB-267268, a nonpeptidic antagonist of alpha(v)beta3 and alpha(v)beta5 integrins, reduces angiogenesis and VEGF expression in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006;47:1600–1605. doi: 10.1167/iovs.05-1314. [DOI] [PubMed] [Google Scholar]
- Witmer AN, Blaauwgeers HG, Weich HA, Alitalo K, Vrensen GF, Schlingemann RO. Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Invest Ophthalmol Vis Sci. 2002;43:849–857. [PubMed] [Google Scholar]
- Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119:1621–1627. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Gong J, Xu Z, Wei Y, Duh EJ. Inhibition of pathological retinal angiogenesis by the integrin alphavbeta3 antagonist tetraiodothyroacetic acid (tetrac) Exp Eye Res. 2012;94:41–48. doi: 10.1016/j.exer.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li C, Baciu PC. Expression of integrins and MMPs during alkaline-burn-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2002;43:955–962. [PubMed] [Google Scholar]