Abstract
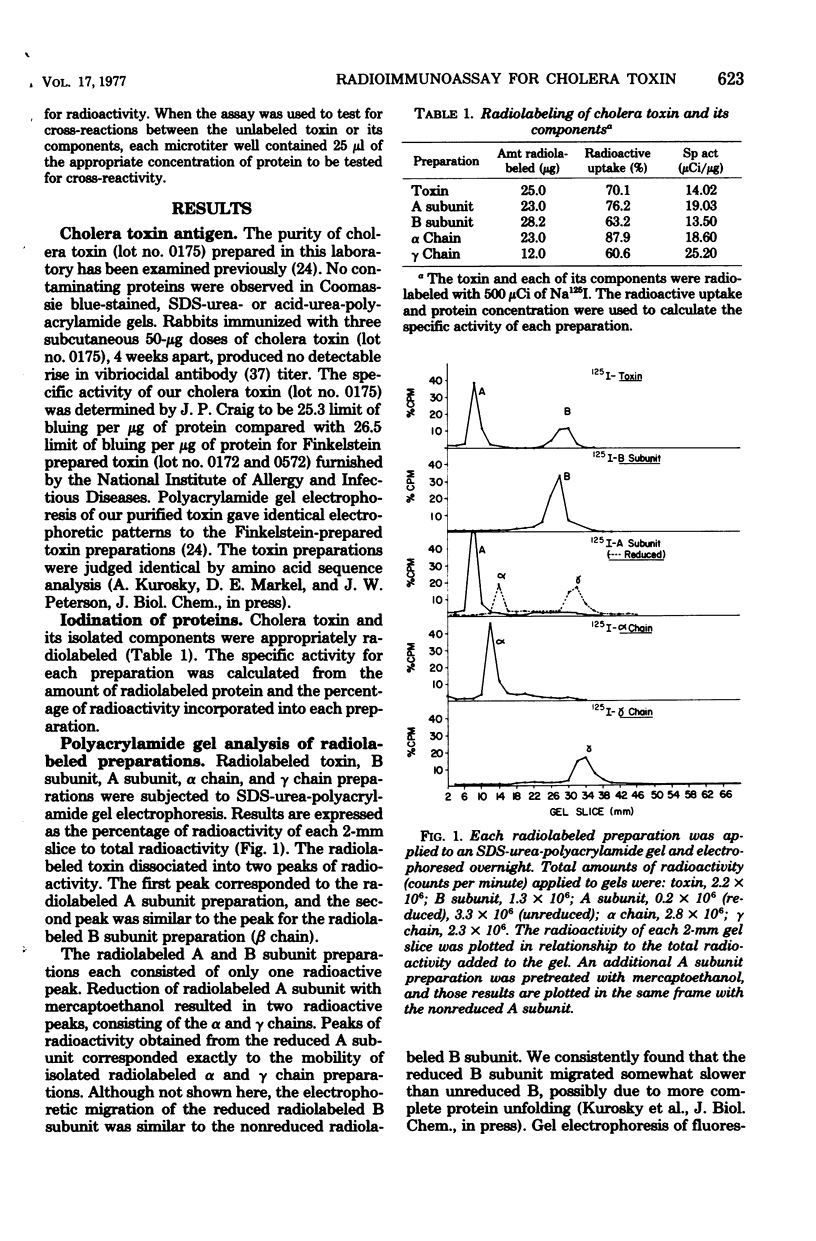
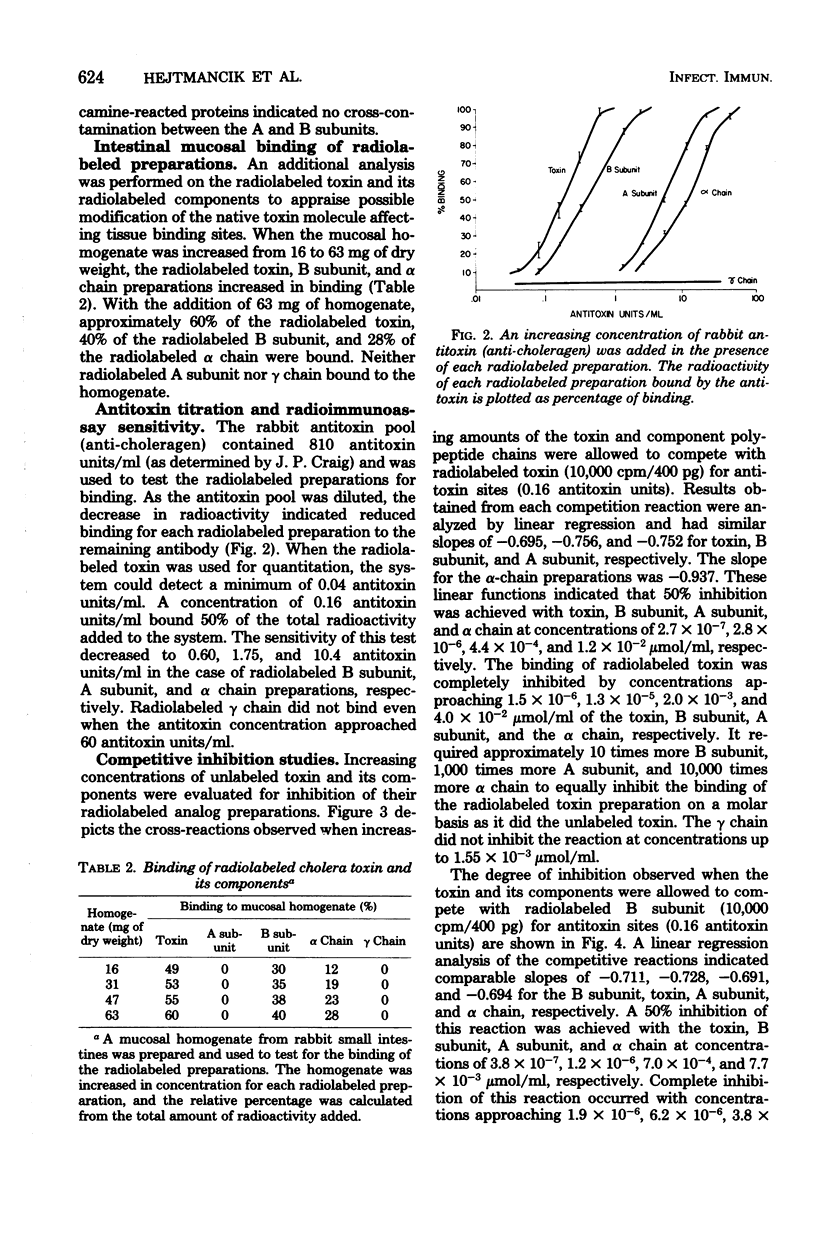
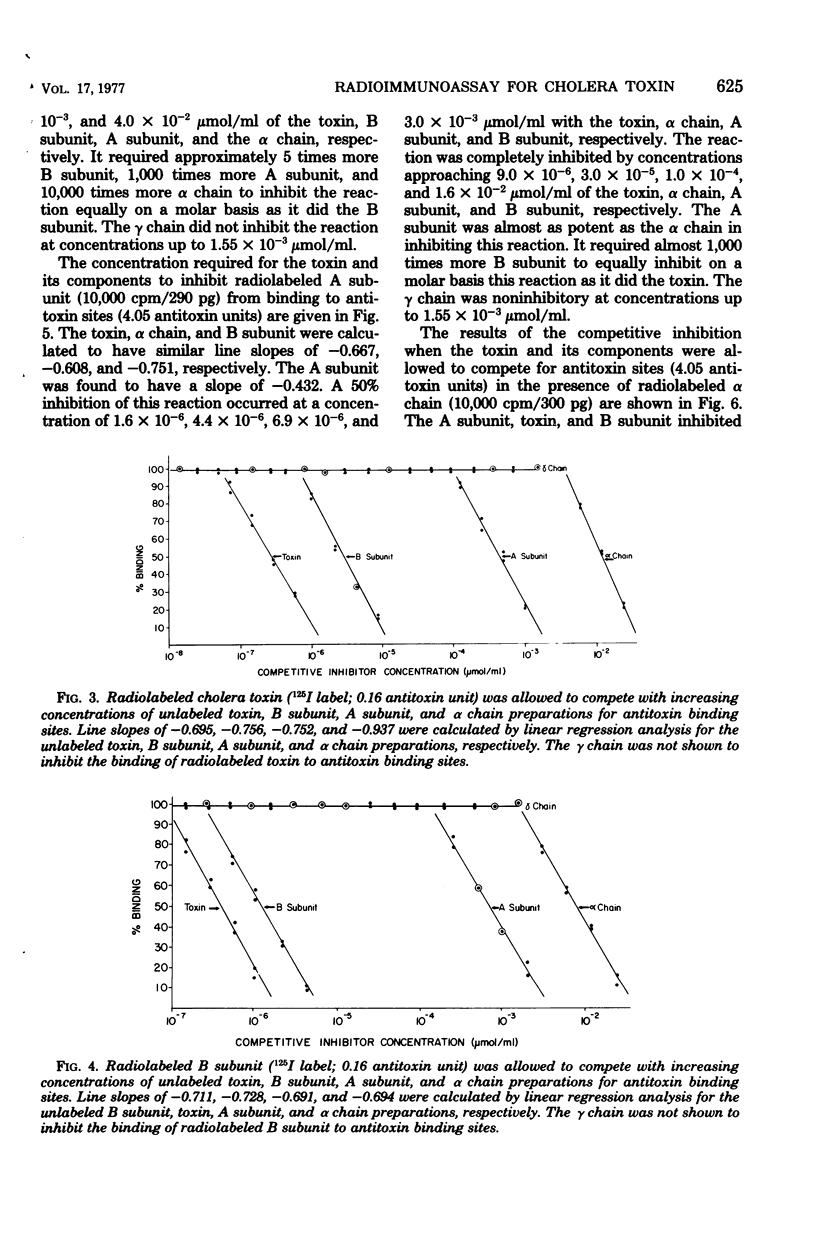
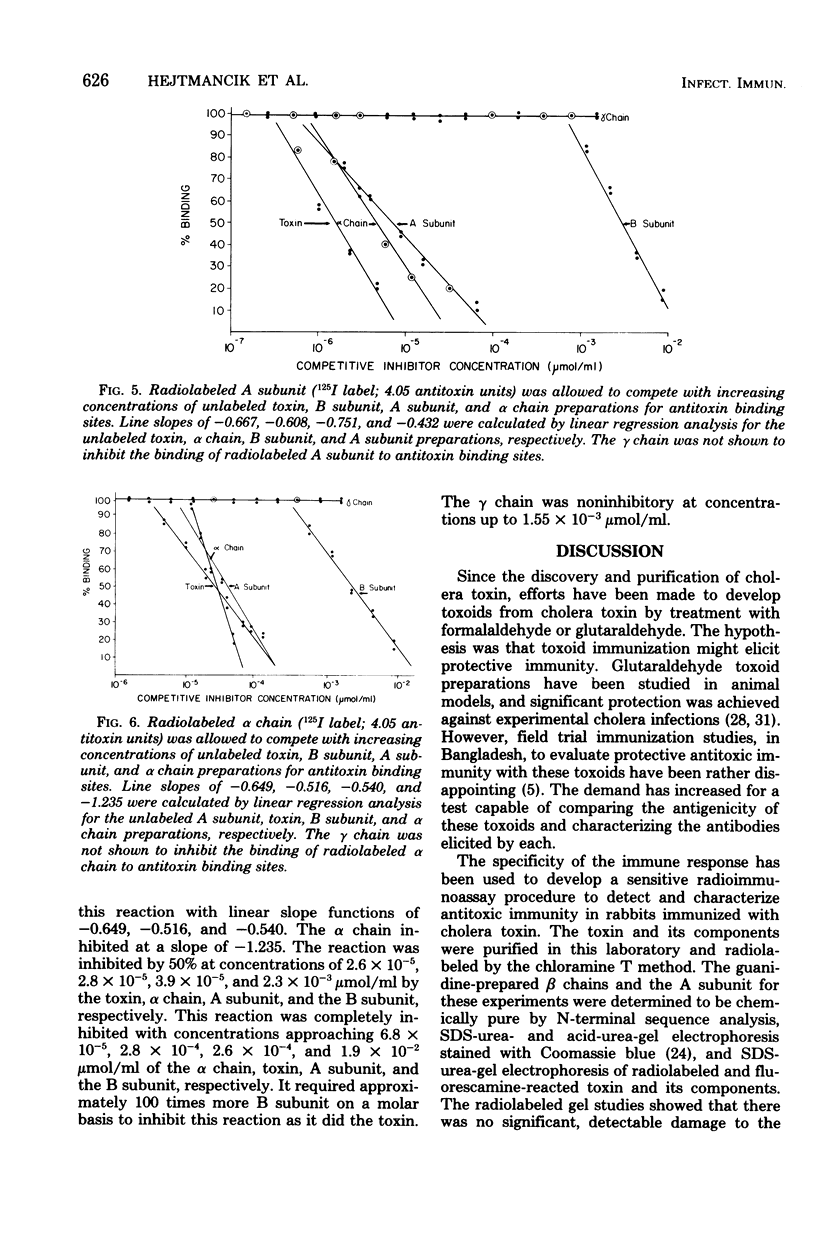
A radioimmunoassay procedure is described for the detection of cholera toxin and its component polypeptide chains. Cholera toxin, A subunit, B subunit, α chain, and γ chain were iodinated by the chloramine T procedure. Radiolabeling did not significantly alter the polyacrylamide electrophoretic migration patterns of the toxin or its components. Moreover, radiolabeled toxin, B subunit, and α chain preparations retained substantial ability to bind to intestinal mucosal homogenates. The minimal amount of antitoxin detectable with radiolabeled toxin was 0.04 antitoxin units/ml. Substitution of radiolabeled B subunit, A subunit, and α chain for radiolabeled toxin decreased the sensitivity of the test. Radiolabeled γ chain did not bind to the antitoxin preparation. Competitive inhibition studies, with titrated anti-choleragen serum and radiolabeled toxin or components, indicated that the minimum concentration of toxin detectable was 7.0 × 10−8 μmol/ml at a 90% inhibition level. The A subunit and α chain preparations inhibited the binding of the radiolabeled B subunit to antitoxin sites. Conversely, B subunit inhibited the binding of radiolabeled A subunit and α chain to antitoxin. The γ chain did not show any reaction with antitoxin or cross-reaction with either whole toxin or its components. These results strongly suggest that the A subunit and the α chain contain antigenic determinant(s) that are common to the B subunit. The B subunit (β chain) and the α chain of cholera toxin may therefore contain region(s) of chemical similarity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman B., Flores J., Witkum P. A., Sharp G. W. Studies on the mode of action of cholera toxin. Effects on solubilized adenylate cyclase. J Clin Invest. 1974 Apr;53(4):1202–1205. doi: 10.1172/JCI107660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C. Experimental cholera in humans. Br Med J. 1966 Jan 15;1(5480):140–142. doi: 10.1136/bmj.1.5480.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Curlin C. T., Mosley W. H., Greenough W. B., 3rd Cholera antitoxin titrations: a comparative study of fat-cell, ileal-loop, and rabbit-skin assays. J Infect Dis. 1973 Mar;127(3):294–298. doi: 10.1093/infdis/127.3.294. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- DE S. N., GHOSE M. L., SEN A. Activities of bacteria-free preparations from Vibrio cholerae. J Pathol Bacteriol. 1960 Apr;79:373–380. doi: 10.1002/path.1700790219. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng P. R., Parkes C. O. SDS electrophoresis of fluorescamine-labeled proteins. Anal Biochem. 1974 May;59(1):323–325. doi: 10.1016/0003-2697(74)90041-4. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., LaRue M. K., LoSpalluto J. J. Properties of the cholera exo-enterotoxin: effects of dispersing agents and reducing agents in gel filtration and electrophoresis. Infect Immun. 1972 Dec;6(6):934–944. doi: 10.1128/iai.6.6.934-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Månsson J. E., Svennerholm L. Tissue receptor for cholera exotoxin: structural requirements of G11 ganglioside in toxin binding and inactivation. Med Biol. 1974 Aug;52(4):229–233. [PubMed] [Google Scholar]
- Kantor H. S. Enterotoxins of Escherichia coli and vibriocholerae: tools for the molecular biologist. J Infect Dis. 1975 May;131 (Suppl):S22–S32. doi: 10.1093/infdis/131.supplement.s22. [DOI] [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W., Fitch W. M. Primary structure of cholera toxin beta-chain: a glycoprotein hormone analog? Science. 1977 Jan 21;195(4275):299–301. doi: 10.1126/science.831277. [DOI] [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Peterson J. W. Tissue-binding properties of the cholera toxin. Infect Immun. 1974 Jul;10(1):157–166. doi: 10.1128/iai.10.1.157-166.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., Verwey W. F. Radiolabeled toxin for studying binding of cholera toxin and toxoids to intestinal mucosal receptor sites. Proc Soc Exp Biol Med. 1974 Apr;145(4):1187–1191. doi: 10.3181/00379727-145-37978. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Mitra R. C., Banwell J. G., Brigham K. L., Fedson D. S., Mondal A. Replacement of water and electrolyte losses in cholera by an oral glucose-electrolyte solution. Ann Intern Med. 1969 Jun;70(6):1173–1181. doi: 10.7326/0003-4819-70-6-1173. [DOI] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]


