Abstract
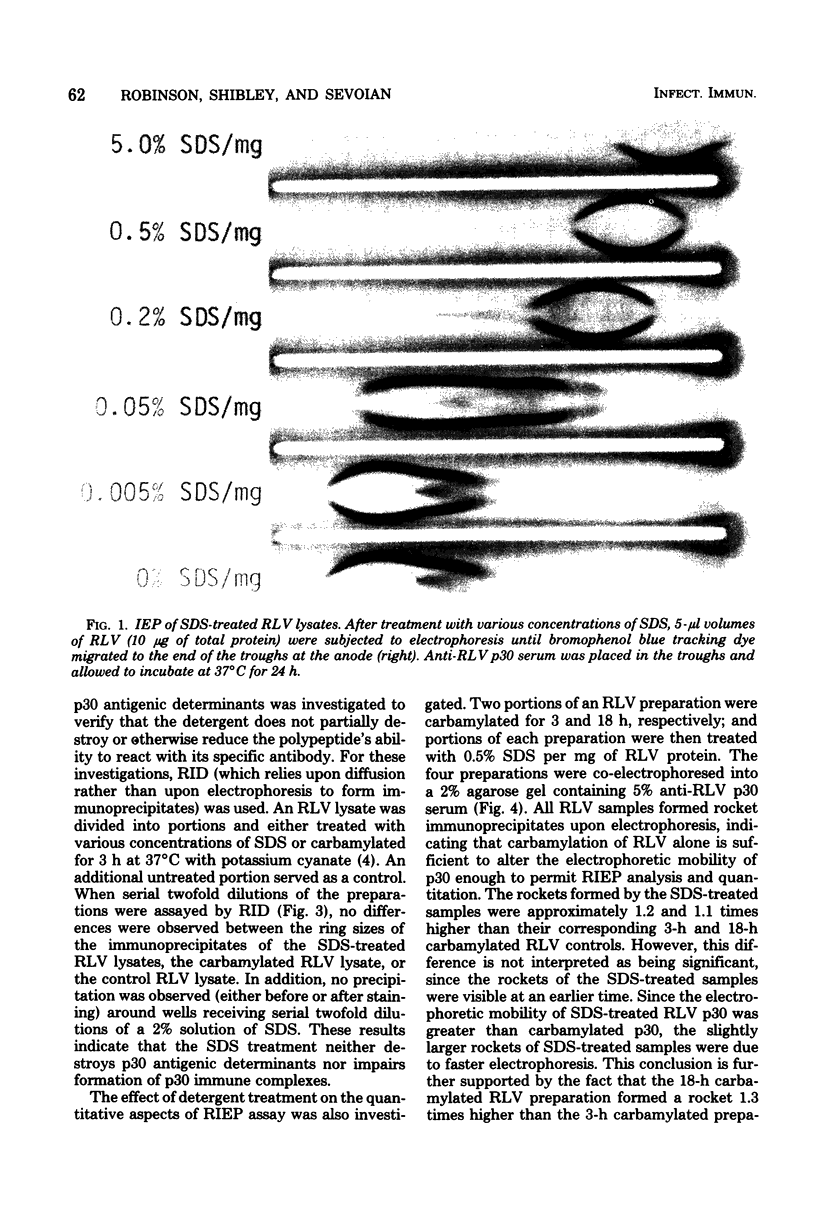
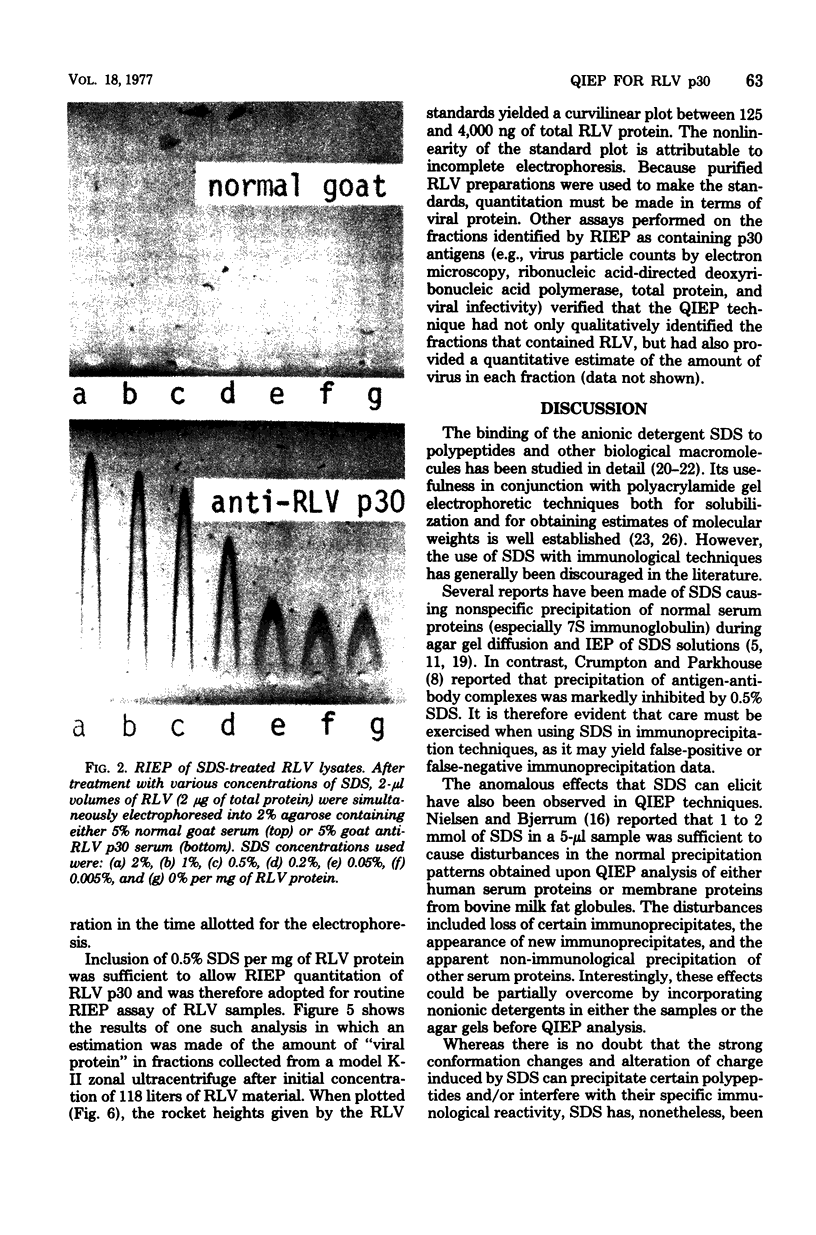
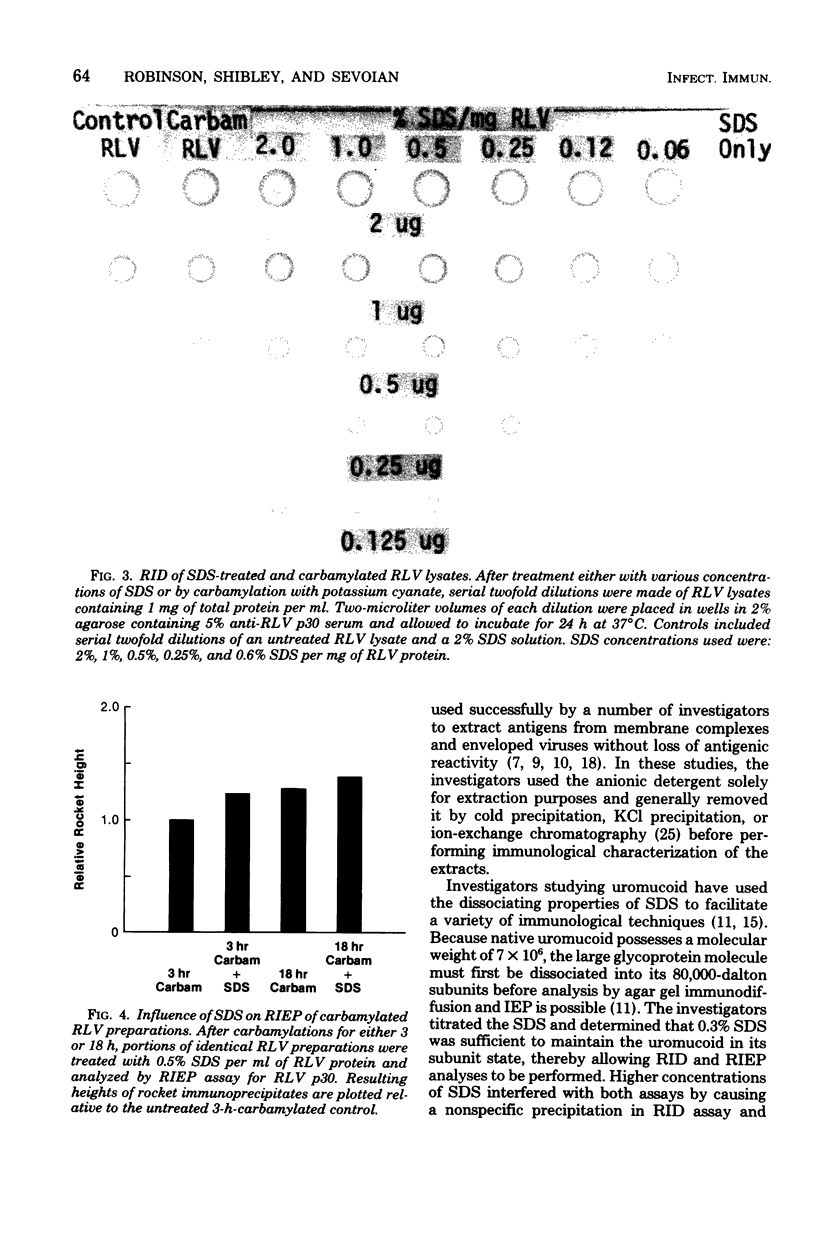
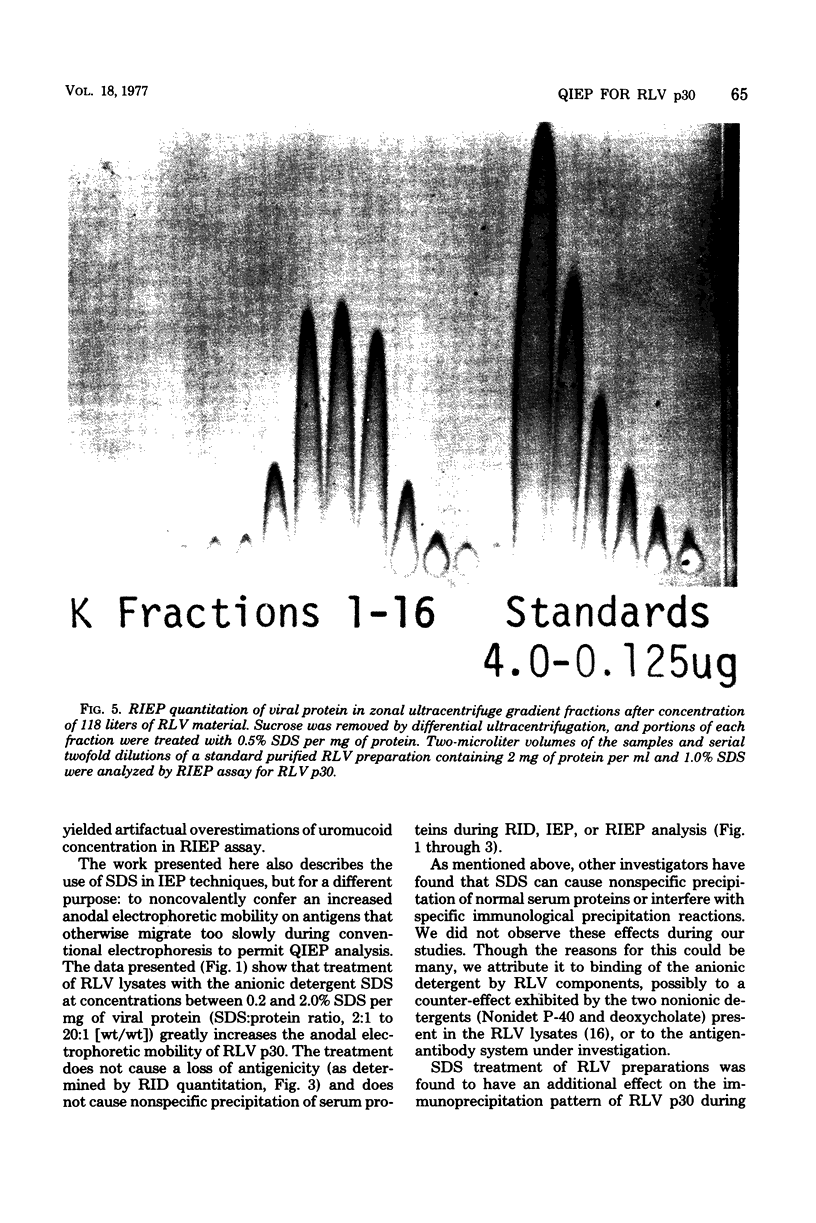
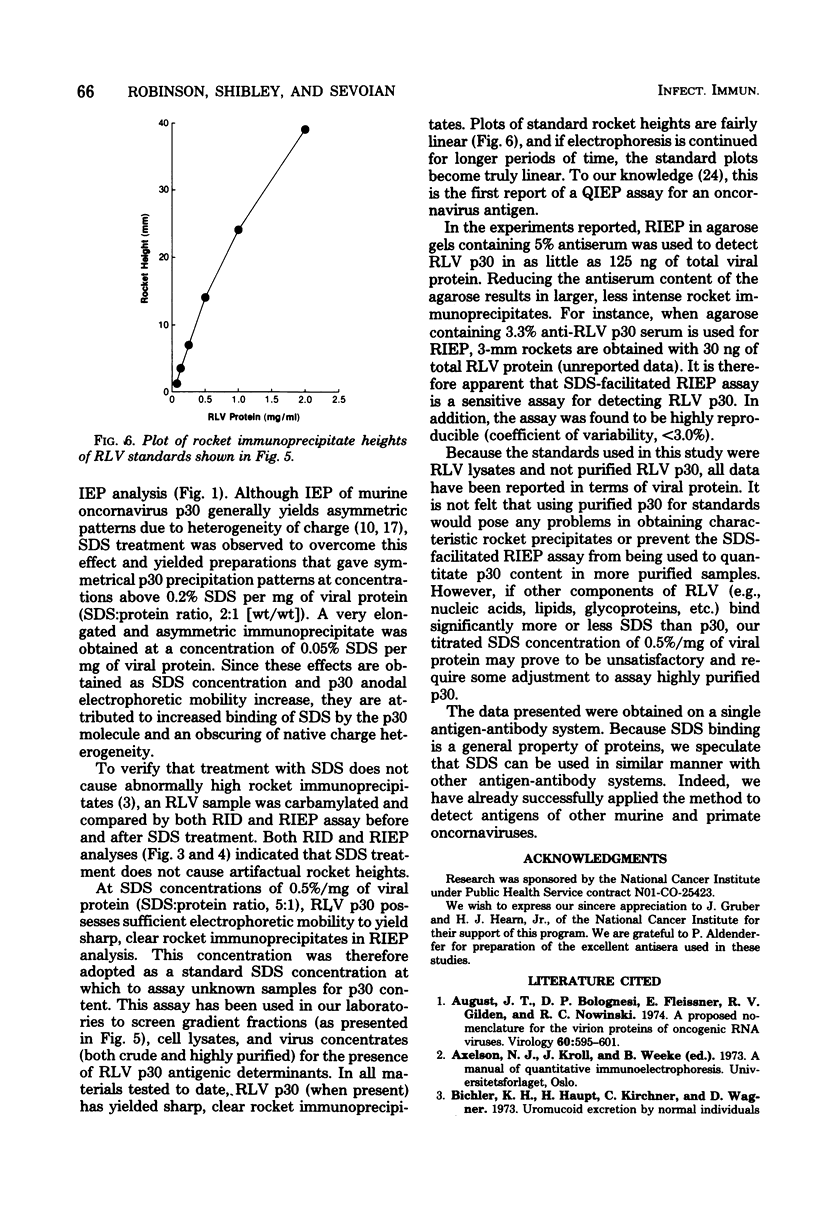
Treatment of Rauscher murine leukemia virus lysates with the anionic detergent sodium dodecyl sulfate (SDS) at concentrations between 0.2 to 2.0% SDS per mg of viral protein greatly increased the anodal electrophoretic mobility of p30, the major internal polypeptide. SDS treatment did not reduce p30 antigenicity or cause nonspecific precipitation of normal serum proteins during subsequent immunoanalysis. The increased anodal electrophoretic mobility allowed assay of Rauscher murine leukemia virus p30 by Laurell rocket immunoelectrophoresis. An SDS-facilitated rocket immunoelectrophoresis assay is described that was highly reproducible (coefficient of variability, less than 3.0%) and capable of detecting 125 ng of viral protein. To our knowledge, this is the first report of a quantitative immunoelectrophoretic assay for an oncornavirus antigen. Since SDS binding is a general property of proteins, this method of noncovalently altering electrophoretic mobility appears to be applicable to other antigen-antibody systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Ingild A., Lowenstein H., Weeke B. Carbamylated antibodies used for quantitation of human IgG. A routine method. Scand J Immunol Suppl. 1973;1:145–148. doi: 10.1111/j.1365-3083.1973.tb03795.x. [DOI] [PubMed] [Google Scholar]
- Cho C. T., Feng K. K. Non-immunological precipitation of serum by sodium dodecyl sulfate in agar diffusion. Appl Microbiol. 1974 Oct;28(4):557–560. doi: 10.1128/am.28.4.557-560.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Corry G., Stone S. S. Antigenic properties of bovine, porcine, and ovine erythrocyte stroma after solubilizaton by sodium dodecyl sulfate and sonification. Immunochemistry. 1969 Jul;6(4):627–632. doi: 10.1016/0019-2791(69)90201-8. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- ECKERT E. A., ROTT R., SCHAEFER W. STUDIES ON THE BAI STRAIN A (AVIAN MYELOBLASTOSIS) VIRUS. II. SOME PROPERTIES OF VIRAL SPLIT PRODUCTS. Virology. 1964 Nov;24:434–440. doi: 10.1016/0042-6822(64)90181-3. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Old L. J. Isolation and some characteristics of a group-specific antigen of the murine leukemia viruses. Virology. 1969 Feb;37(2):189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. W., Perry A., Robinson O. R., Jr, Shibley G. P. Method for reproducible large-volume production and purification of Rauscher murine leukemia virus. Appl Environ Microbiol. 1976 Feb;31(2):182–188. doi: 10.1128/aem.31.2.182-188.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Marr A. M., Neuberger A., Ratcliffe W. A. Rabbit Tamm-Horsfall urinary glycoprotein. Chemical composition and subunit structure. Biochem J. 1971 May;122(5):623–631. doi: 10.1042/bj1220623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Fisher C. L., Stanley T. B., Gilden R. V. Proteins of the murine C-type RNA tumour viruses: isolation of a group-specific antigen by isoelectric focusing. J Gen Virol. 1970 Jul;8(1):1–10. doi: 10.1099/0022-1317-8-1-1. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Summers M. R., Foreman C., Gilden R. V. Murine type-C virus group-specific antigens: interstrain immunochemical, biophysical, and amino acid sequence differences. J Virol. 1974 Dec;14(6):1559–1574. doi: 10.1128/jvi.14.6.1559-1574.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Hierholzer J. C., Ziegler D. W. Nonspecific precipitation of serum proteins by sodium lauryl sulfate in agar diffusion and immunoelectrophoresis. Appl Microbiol. 1971 May;21(5):903–906. doi: 10.1128/am.21.5.903-906.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Verbruggen Quantitative immunoelectrophoretic methods: a literature survey. Clin Chem. 1975 Jan;21(1):5–43. [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeke B. Carbamylated human immunoglobulins tested by electrophoresis in agarose and antibody containing agarose. Scand J Clin Lab Invest. 1968;21(4):351–354. doi: 10.3109/00365516809077006. [DOI] [PubMed] [Google Scholar]