Abstract
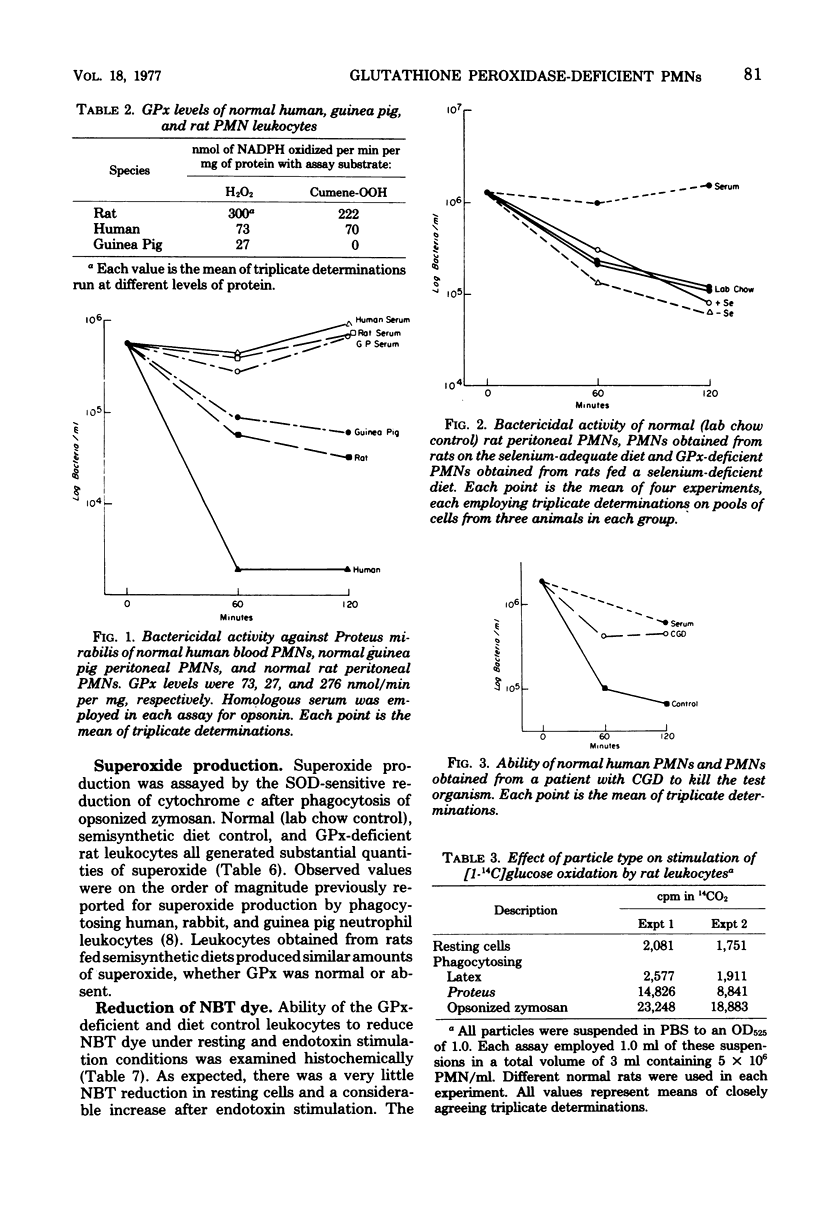
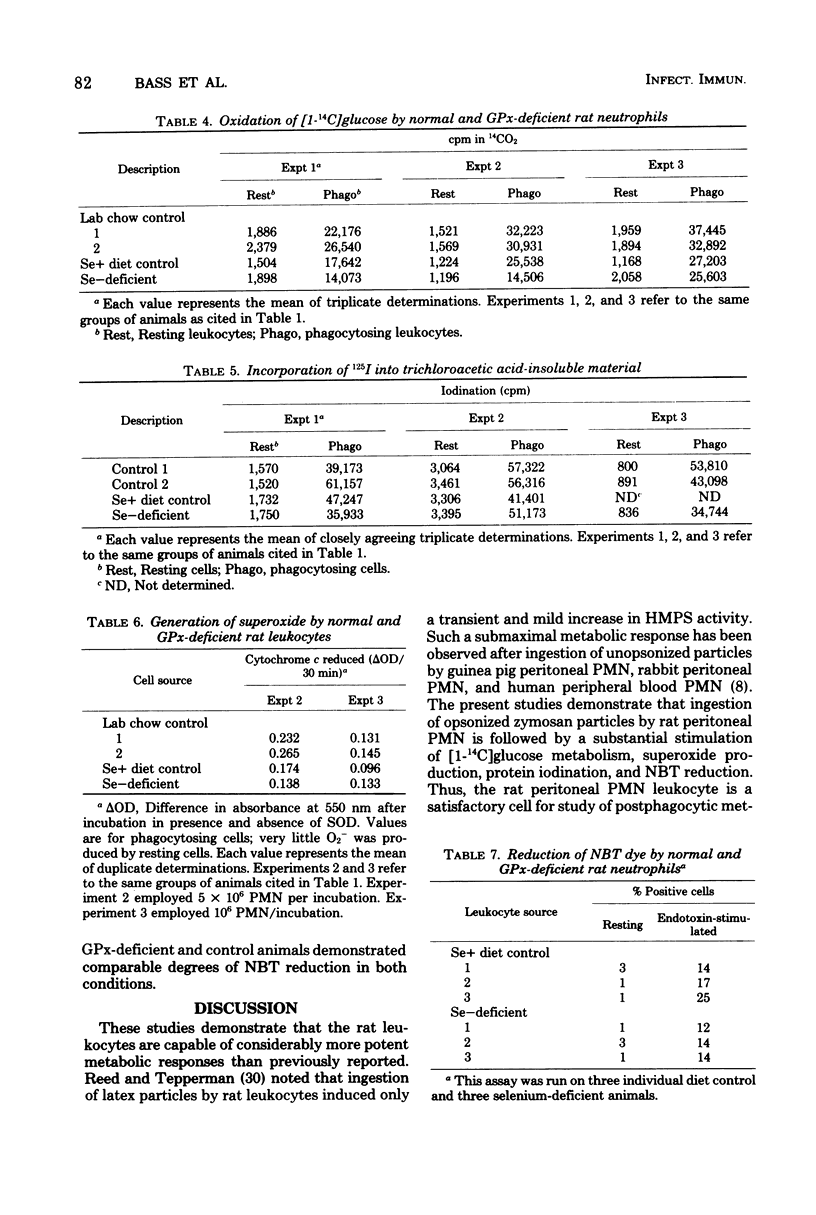
Glutathione peroxidase (GPx) deficiency has been proposed as a cause of some instances of chronic granulomatous disease (CGD). GPx activity varies greatly among species, and specific deficiency of this selenium-dependent enzyme can be produced by dietary selenium deficiency in rats. Bactericidal activity of polymorphonuclear (PMN) leukocytes from normal rats, humans, and guinea pigs (GPx high, intermediate, and nearly absent, respectively), selenium-deficient rats (GPx absent), and a patient with CGD were compared. There was no correlation between natural levels of GPx and bactericidal activity; only CGD was associated with inability to kill a Proteus mirabilis strain in vitro (killing known to be dependent on oxidative mechanisms). Postphagocytic metabolism was examined in normal and GPx-deficient rats. Both demonstrated normal iodination and superoxide production during phagocytosis and gave similar histochemical reduction of nitroblue tetrazolium dye under either resting or endotoxin-stimulation conditions. Postphagocytic hexose monophosphate shunt activity was somewhat lower in PMN from GPx-deficient animals as compared with normal but was substantially (10-fold) higher than that observed in resting cells. Thus, postphagocytic oxidative responses and subsequent bactericidal activity of PMN leukocytes were not compromised by complete absence of GPx, even in the species with the highest natural level of this enzyme. These results are not compatible with the hypothesis that CGD can be caused by a deficiency of GPx.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Masters B. S. Some effects of selenium deficiency on the hepatic microsomal cytochrome P-450 system in the rat. Arch Biochem Biophys. 1975 Sep;170(1):124–131. doi: 10.1016/0003-9861(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., LaVia M. F., Spurr C. L., Baehner R. L. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. 1972 Apr;51(4):769–778. doi: 10.1172/JCI106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Mulikin D., McCall C. E. The generation of superoxide anion by various types of phagocyte. J Infect Dis. 1975 Apr;131(4):443–446. doi: 10.1093/infdis/131.4.443. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C. Normal leukocyte glutathione peroxidase activity in patients with chronic granulomatous disease. J Pediatr. 1976 Oct;89(4):598–600. doi: 10.1016/s0022-3476(76)80396-4. [DOI] [PubMed] [Google Scholar]
- Dechatelet L. R., Cooper M. R., McCall C. E. Dissociation by colchicine of the hexose monophosphate shunt activation from the bactericidal activity of the leukocyte. Infect Immun. 1971 Jan;3(1):66–72. doi: 10.1128/iai.3.1.66-72.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., Sunde R. A., Hoekstra W. G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974 May;104(5):580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Jandl J. H. Effects of sulfhydryl inhibition on red blood cells. 3. Glutathione in the regulation of the hexose monophosphate pathway. J Biol Chem. 1966 Sep 25;241(18):4243–4250. [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- Matsuda I., Oka Y., Taniguchi N., Furuyama M., Kodama S., Arashima S., Mitsuyama T. Leukocyte glutathione peroxidase deficiency in a male patient with chronic granulomatous disease. J Pediatr. 1976 Apr;88(4 Pt 1):581–583. doi: 10.1016/s0022-3476(76)80010-8. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Qualliotine D., DeChatelet L. R., McCall C. E., Cooper M. R. Effect of catecholamines on the bactericidal activity of polymorphonuclear leukocytes. Infect Immun. 1972 Sep;6(3):211–217. doi: 10.1128/iai.6.3.211-217.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Reed P. W., Tepperman J. Phagocytosis-associated metabolism and enzymes in the rat polymorphonuclear leukocyte. Am J Physiol. 1969 Feb;216(2):223–230. doi: 10.1152/ajplegacy.1969.216.2.223. [DOI] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest. 1976 Nov;58(5):1174–1184. doi: 10.1172/JCI108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J. C., Kerr M. M., Richards I. D., Hutchison J. H. Measurements of oxygen tension in subcutaneous tissues of newborn infants under normobaric and hyperbaric conditions. Lancet. 1968 Aug 3;2(7562):232–236. doi: 10.1016/s0140-6736(68)92349-0. [DOI] [PubMed] [Google Scholar]
- Serfass R. E., Ganther H. E. Defective microbicidal activity in glutathione peroxidase-deficient neutrophils of selenium-deficient rats. Nature. 1975 Jun 19;255(5510):640–641. doi: 10.1038/255640a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Barnett J. A., Sanford J. P. Heterogeneity of immune response to the somatic (O) antigens of Proteus mirabilis. J Immunol. 1970 Aug;105(2):404–410. [PubMed] [Google Scholar]


