Abstract
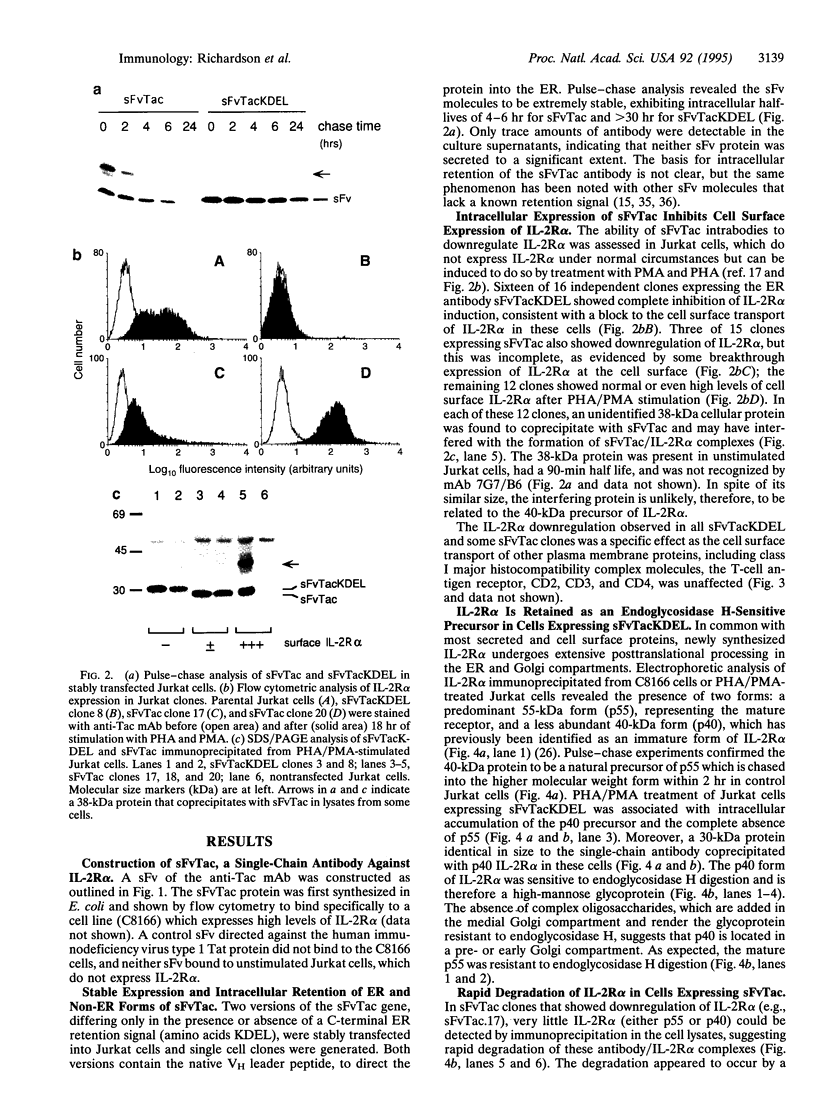
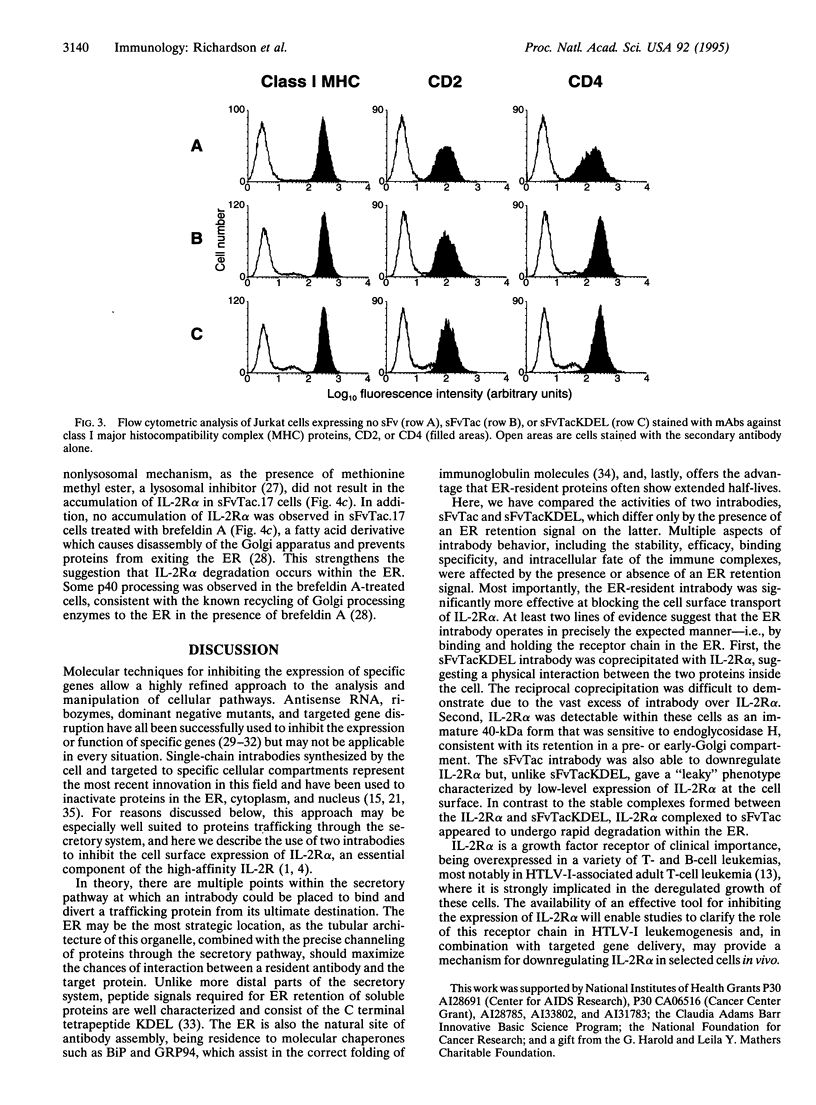
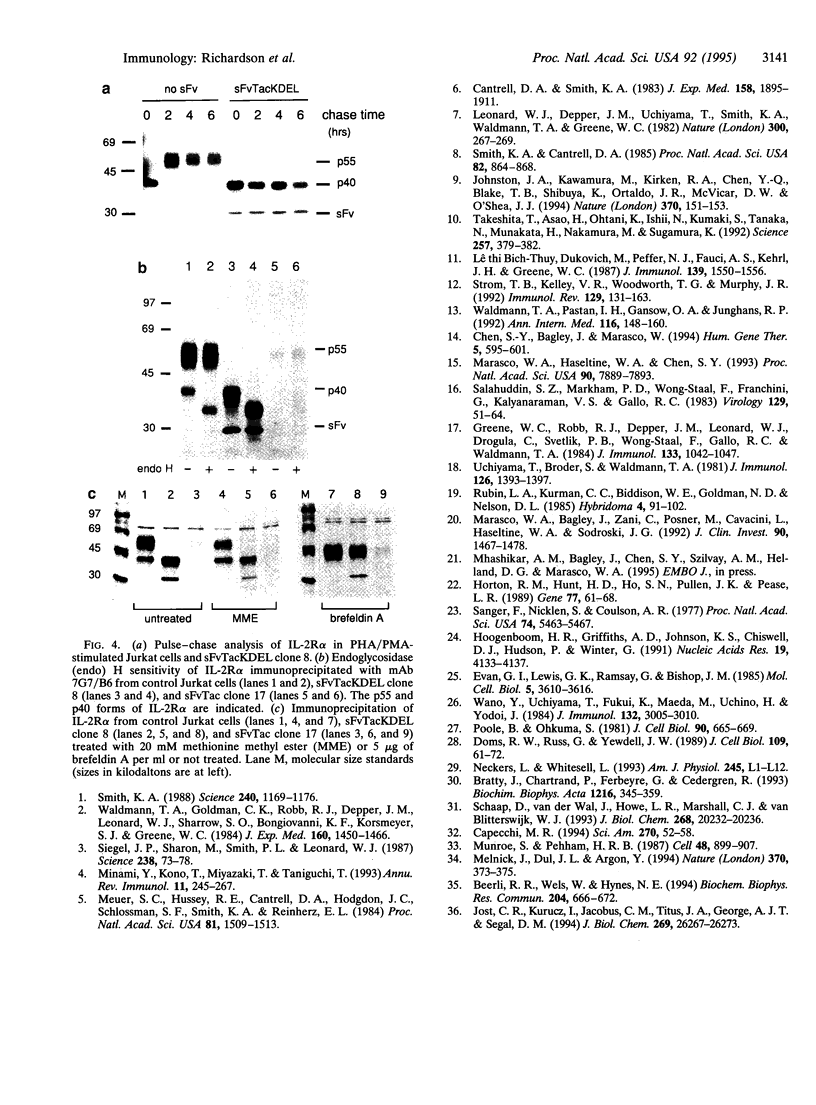
The experimental manipulation of peptide growth hormones and their cellular receptors is central to understanding the pathways governing cellular signaling and growth control. Previous work has shown that intracellular antibodies targeted to the endoplasmic reticulum (ER) can be used to capture specific proteins as they enter the ER, preventing their transport to the cell surface. Here we have used this technology to inhibit the cell surface expression of the alpha subunit of the high-affinity interleukin 2 receptor (IL-2R alpha). A single-chain variable-region fragment of the anti-Tac monoclonal antibody was constructed with a signal peptide and a C-terminal ER retention signal. Intracellular expression of the single-chain antibody was found to completely abrogate cell surface expression of IL-2R alpha in stimulated Jurkat T cells. IL-2R alpha was detectable within the Jurkat cells as an immature 40-kDa form that was sensitive to endoglycosidase H, consistent with its retention in a pre- or early Golgi compartment. A single-chain antibody lacking the ER retention signal was also able to inhibit cell surface expression of IL-2R alpha although the mechanism appeared to involve rapid degradation of the receptor chain within the ER. These intracellular antibodies will provide a valuable tool for examining the role of IL-2R alpha in T-cell activation, IL-2 signal transduction, and the deregulated growth of leukemic cells which overexpress IL-2R alpha.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerli R. R., Wels W., Hynes N. E. Autocrine inhibition of the epidermal growth factor receptor by intracellular expression of a single-chain antibody. Biochem Biophys Res Commun. 1994 Oct 28;204(2):666–672. doi: 10.1006/bbrc.1994.2511. [DOI] [PubMed] [Google Scholar]
- Bich-Thuy L. T., Dukovich M., Peffer N. J., Fauci A. S., Kehrl J. H., Greene W. C. Direct activation of human resting T cells by IL 2: the role of an IL 2 receptor distinct from the Tac protein. J Immunol. 1987 Sep 1;139(5):1550–1556. [PubMed] [Google Scholar]
- Bratty J., Chartrand P., Ferbeyre G., Cedergren R. The hammerhead RNA domain, a model ribozyme. Biochim Biophys Acta. 1993 Dec 14;1216(3):345–359. doi: 10.1016/0167-4781(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Targeted gene replacement. Sci Am. 1994 Mar;270(3):52–59. doi: 10.1038/scientificamerican0394-52. [DOI] [PubMed] [Google Scholar]
- Chen S. Y., Bagley J., Marasco W. A. Intracellular antibodies as a new class of therapeutic molecules for gene therapy. Hum Gene Ther. 1994 May;5(5):595–601. doi: 10.1089/hum.1994.5.5-595. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Depper J. M., Leonard W. J., Drogula C., Svetlik P. B., Wong-Staal F., Gallo R. C., Waldmann T. A. Phorbol diester induces expression of Tac antigen on human acute T lymphocytic leukemic cells. J Immunol. 1984 Aug;133(2):1042–1047. [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Jost C. R., Kurucz I., Jacobus C. M., Titus J. A., George A. J., Segal D. M. Mammalian expression and secretion of functional single-chain Fv molecules. J Biol Chem. 1994 Oct 21;269(42):26267–26273. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Bagley J., Zani C., Posner M., Cavacini L., Haseltine W. A., Sodroski J. Characterization of the cDNA of a broadly reactive neutralizing human anti-gp120 monoclonal antibody. J Clin Invest. 1992 Oct;90(4):1467–1478. doi: 10.1172/JCI116014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Haseltine W. A., Chen S. Y. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7889–7893. doi: 10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J., Dul J. L., Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994 Aug 4;370(6488):373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985 Summer;4(2):91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., van der Wal J., Howe L. R., Marshall C. J., van Blitterswijk W. J. A dominant-negative mutant of raf blocks mitogen-activated protein kinase activation by growth factors and oncogenic p21ras. J Biol Chem. 1993 Sep 25;268(27):20232–20236. [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Kelley V. R., Woodworth T. G., Murphy J. R. Interleukin-2 receptor-directed immunosuppressive therapies: antibody- or cytokine-based targeting molecules. Immunol Rev. 1992 Oct;129:131–163. doi: 10.1111/j.1600-065x.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Pastan I. H., Gansow O. A., Junghans R. P. The multichain interleukin-2 receptor: a target for immunotherapy. Ann Intern Med. 1992 Jan 15;116(2):148–160. doi: 10.7326/0003-4819-116-2-148. [DOI] [PubMed] [Google Scholar]
- Wano Y., Uchiyama T., Fukui K., Maeda M., Uchino H., Yodoi J. Characterization of human interleukin 2 receptor (Tac antigen) in normal and leukemic T cells: co-expression of normal and aberrant receptors on Hut-102 cells. J Immunol. 1984 Jun;132(6):3005–3010. [PubMed] [Google Scholar]