Abstract
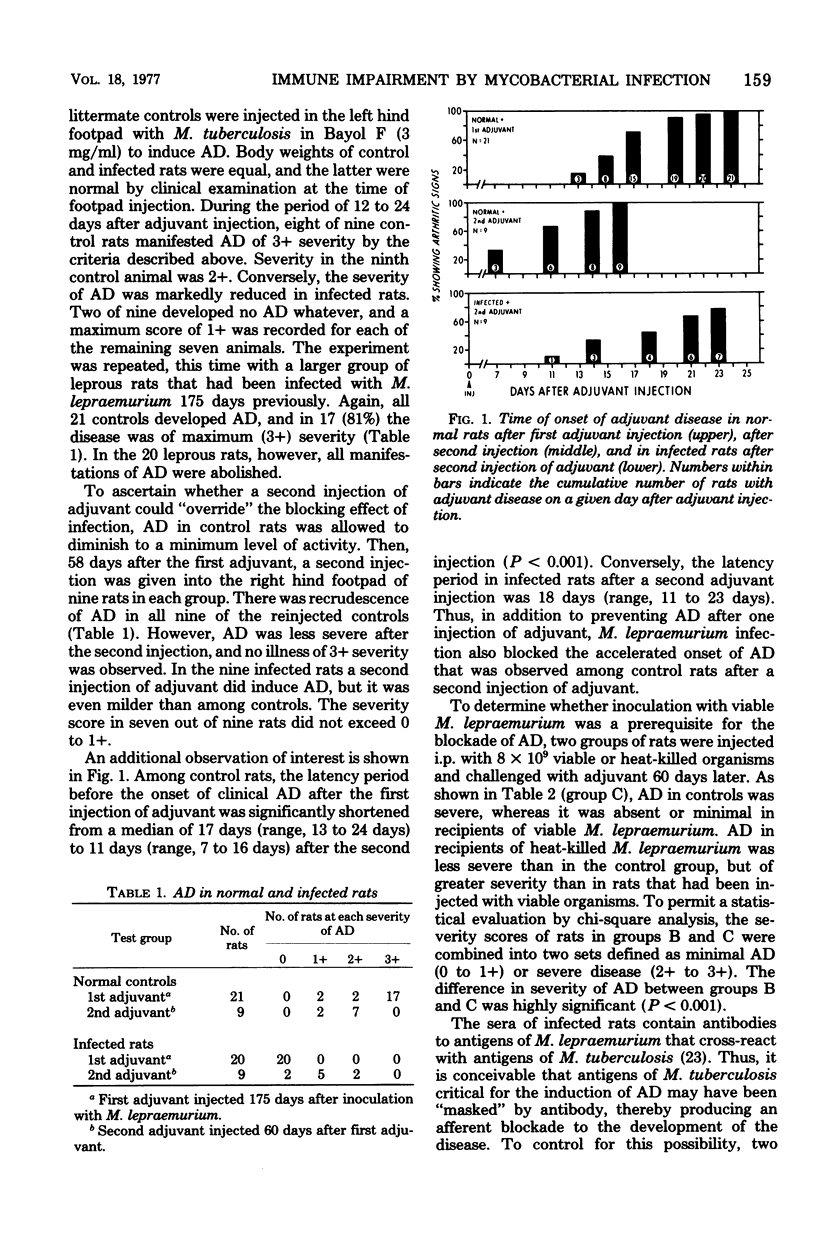
The effect of chronic infection with Mycobacterium lepraemurium upon cell-mediated immune responses was studied in Lewis rats. Rats infected for 40 to 175 days were completely protected from attempted induction of experimental adjuvant disease, and the severity of experimental allergic encephalomyelitis in leprous rats was markedly attenuated. Full manifestations of each autoimmune disease were expressed in littermate control groups. Skin homograft rejection by infected rats was significantly impaired (P less than 0.001) as was the delayed-type hypersensitivity response to sheep erythrocytes (P less than 0.02). It is suggested that chronic infection with M. lepraemurium exerts a nonspecific inhibitory effect on cell-mediated immunity by perturbation of normal lymphocyte recirculation and by induction of immuno-suppressor cell activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allt G., Evans E. M., Evans D. H., Targett G. A. Effect of infection with trypanosomes on the development of experimental allergic neuritis in rabbits. Nature. 1971 Sep 17;233(5316):197–199. doi: 10.1038/233197b0. [DOI] [PubMed] [Google Scholar]
- Allwood G. G., Asherson G. L. Depression of delayed hypersensitivity by pretreatment with Freund-type adjuvants. 3. Depressed arrival of lymphoid cells at recently immunized lymph nodes in mice pretreated with adjuvants. Clin Exp Immunol. 1972 Aug;11(4):579–584. [PMC free article] [PubMed] [Google Scholar]
- Axelrad M. A. Suppression of delayed hypersensitivity by antigen and antibody. Is a common precursor cell responsible for both delayed hypersensitivity and antibody formation? Immunology. 1968 Aug;15(2):159–171. [PMC free article] [PubMed] [Google Scholar]
- Beiguelman B. Leprosy and genetics. A review of past research with remarks concerning future investigations. Bull World Health Organ. 1967;37(3):461–476. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- CUNNINGHAM V. R., FIELD E. J. EXPERIMENTAL ALLERGIC ENCEPHALOMYELITIS: PROTECTION EXPERIMENTS WITH ENCEPHALITOGENIC FACTOR AND TUBERCLE FRACTIONS. Ann N Y Acad Sci. 1965 Mar 31;122:346–355. doi: 10.1111/j.1749-6632.1965.tb20219.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- Cooperband S. R., Badger A. M., Davis R. C., Schmid K., Mannick J. A. The effect of immunoregulatory globulin (IRA) upon lymphocytes in vitro. J Immunol. 1972 Jul;109(1):154–163. [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Koller P. C. The morphology of immune reactions in normal, thymectomized and reconstituted mice. I. The response to sheep erythrocytes. Immunology. 1969 Jan;16(1):57–69. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. I. Thymic dependency. J Immunol. 1971 Jun;106(6):1524–1531. [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Studies of the mechanism whereby adjuvant disease is suppressed in rats pretreated with mycobacteria. Int Arch Allergy Appl Immunol. 1967;31(1):57–68. doi: 10.1159/000229854. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Howard J. C. Inhibition of experimental allergic encephalomyelitis in rats severely depleted of T cells. Science. 1974 Nov 29;186(4166):839–841. doi: 10.1126/science.186.4166.839. [DOI] [PubMed] [Google Scholar]
- ISAKOVIC K., WAKSMAN B. H. EFFECT OF SENSITIZATION TO BSA ON ADJUVANT DISEASE IN NORMAL AND NEONATALLY THYMECTOMIZED RATS. Proc Soc Exp Biol Med. 1965 Jul;119:676–678. doi: 10.3181/00379727-119-30269. [DOI] [PubMed] [Google Scholar]
- JANKOVIC B. D. Impairment of immunological reactivity in guinea pigs by prior injection of adjuvant. Nature. 1962 Feb 24;193:789–790. doi: 10.1038/193789a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- LUMSDEN C. E. THE PROBLEM OF PREVENTION AND CONTROL OF AUTO-ALLERGIC INFLAMMATION IN THE NERVOUS SYSTEM. Z Immunitats Allergieforsch. 1964 Mar;126:209–216. [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Rea T. H., Levan N. E., Terasaki P. I. Histocompatibility antigens in patients with leprosy. J Infect Dis. 1976 Dec;134(6):615–618. doi: 10.1093/infdis/134.6.615. [DOI] [PubMed] [Google Scholar]
- Reinisch C. L., Gleiner N. A., Schlossman S. F. Adjuvant regulation of T cell function. J Immunol. 1976 Mar;116(3):710–715. [PubMed] [Google Scholar]
- Salzano F. M. Blood groups and leprosy. J Med Genet. 1967 Jun;4(2):102–106. doi: 10.1136/jmg.4.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Depression of delayed-type hypersensitivity by Corynebacterium parvum: mandatory role of the spleen. Cell Immunol. 1974 Aug;13(2):251–263. doi: 10.1016/0008-8749(74)90243-3. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Smith J. B., McIntosh G. H., Morris B. The migration of cells through chronically inflamed tissues. J Pathol. 1970 Jan;100(1):21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- Uberoi S., Malaviya A. N., Chattopadhyay C., Kumar R., Shrinivas Secondary immunodeficiency in miliary tuberculosis. Clin Exp Immunol. 1975 Dec;22(3):404–408. [PMC free article] [PubMed] [Google Scholar]
- Vacher J., Deraedt R., Benzoni J. Influence of a change in the activity of the reticuloendothelial system (RES) on the development of adjuvant-induced polyarthritis in the rat. J Reticuloendothel Soc. 1973 Jun;13(6):579–588. [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- Whitehouse D. J., Whitehouse M. W., Pearson C. M. Passive transfer of adjuvant-induced arthritis and allergic encephalomyelitis in rats using thoracic duct lymphocytes. Nature. 1969 Dec 27;224(5226):1322–1322. doi: 10.1038/2241322a0. [DOI] [PubMed] [Google Scholar]
- Zatz M. M. Effects of BCG on lymphocyte trapping. J Immunol. 1976 Jun;116(6):1587–1591. [PubMed] [Google Scholar]


