Abstract
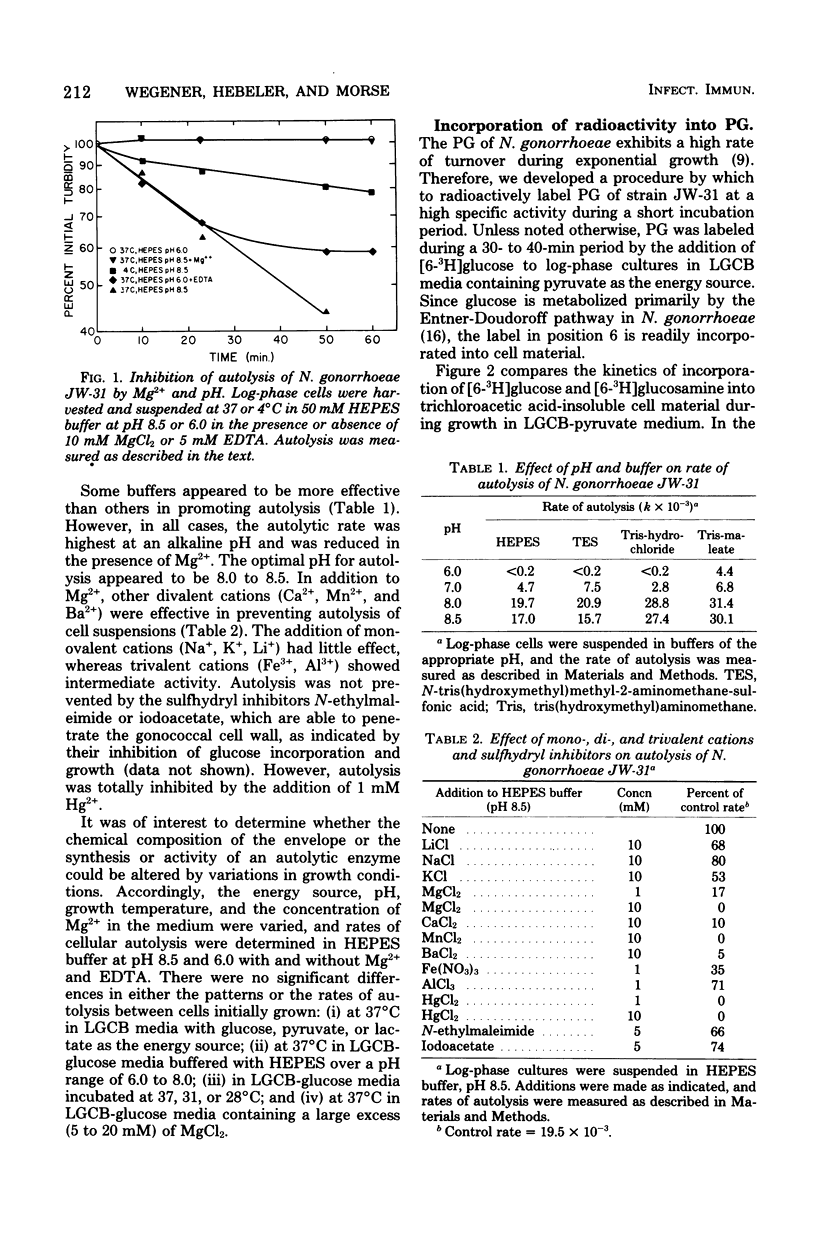
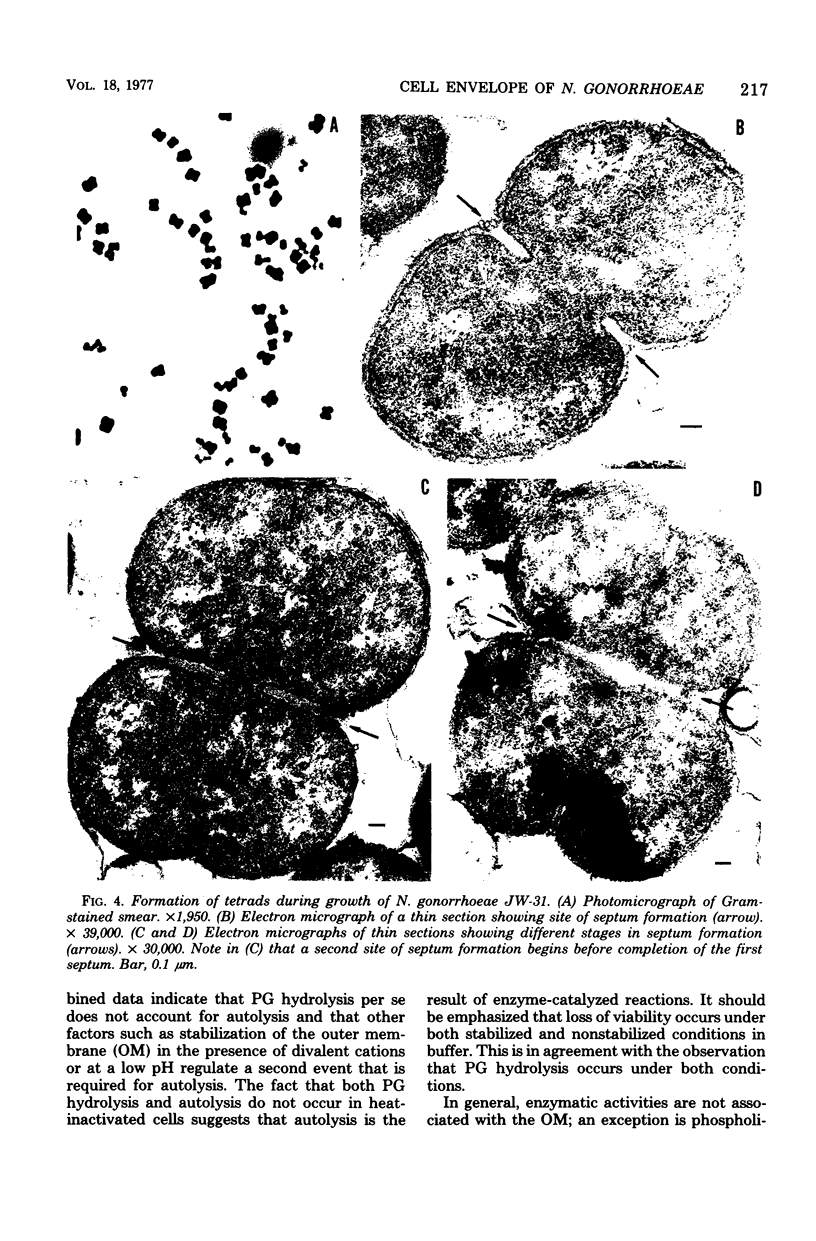
Neisseria gonorrhoeae readily underwent autolysis when suspended in N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer at alkaline pH values. Autolysis was inhibited by the addition of Mg2+ or other divalent cations. Autolysis was also suppressed at acid pH (pH 6.0). Suspension of cells in buffer was accompanied by the hydrolysis of peptidoglycan. The rate of peptidoglycan hydrolysis in HEPES buffer was maximal at pH 8.5 and was similar in the presence or absence of Mg2+. Therefore, divalent cation stabilization against autolysis is not mediated by inhibition of peptidoglycan hydrolysis. Peptidoglycan hydrolysis occurred in HEPES buffer (pH 6.0), but at a rate that was 50% of the maximum. Incubation of cells with chloramphenicol or rifampin before suspension in HEPES buffer (pH 8.5) partially prevented autolysis; under these conditions, peptidoglycan hydrolysis still occurred, but at a reduced rate. Old and new peptidoglycans were hydrolyzed at similar rates. Peptidoglycan hydrolysis results in solubilization of both the peptide and glycan moieties.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Elmros T., Burman L. G., Bloom G. D. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 May;126(2):969–976. doi: 10.1128/jb.126.2.969-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmros T., Sandström G., Burman L. Autolysis of Neisseria gonorrhoeae. Relation between mechanical stability and viability. Br J Vener Dis. 1976 Aug;52(4):246–249. doi: 10.1136/sti.52.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Narrod S., Wong W., Young F. E., Chatterjee A. N. Autolysis in Staphylococcus aureus: preferential release of old cell walls. J Bacteriol. 1974 Sep;119(3):672–676. doi: 10.1128/jb.119.3.672-676.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Senff L. M., Wegener W. S., Brooks G. F., Finnerty W. R., Makula R. A. Phospholipid composition and phospholipase A activity of Neisseria gonorrhoeae. J Bacteriol. 1976 Aug;127(2):874–880. doi: 10.1128/jb.127.2.874-880.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Elmros T., Normark S., Winblad B. Growth pattern and cell division in Neisseria gonorrhoeae. J Bacteriol. 1977 Jan;129(1):333–342. doi: 10.1128/jb.129.1.333-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



