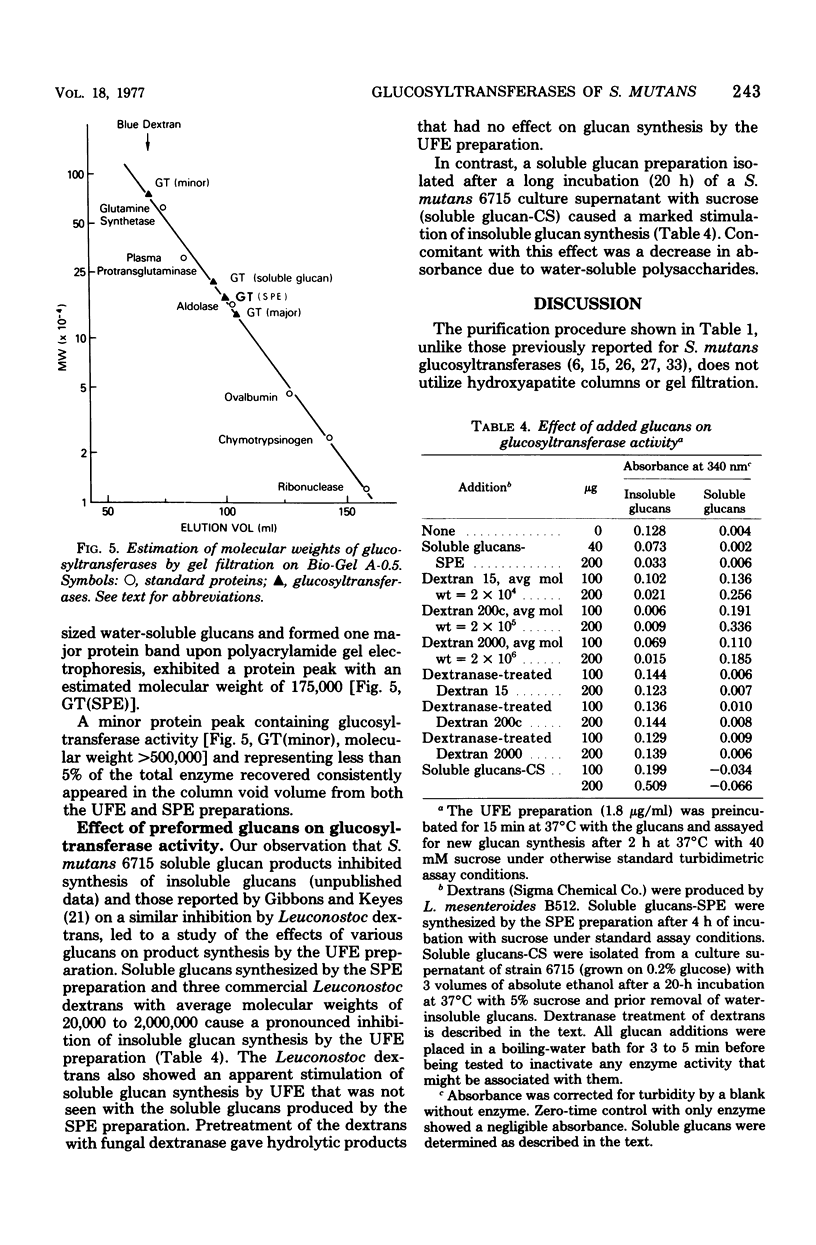
Abstract
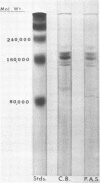
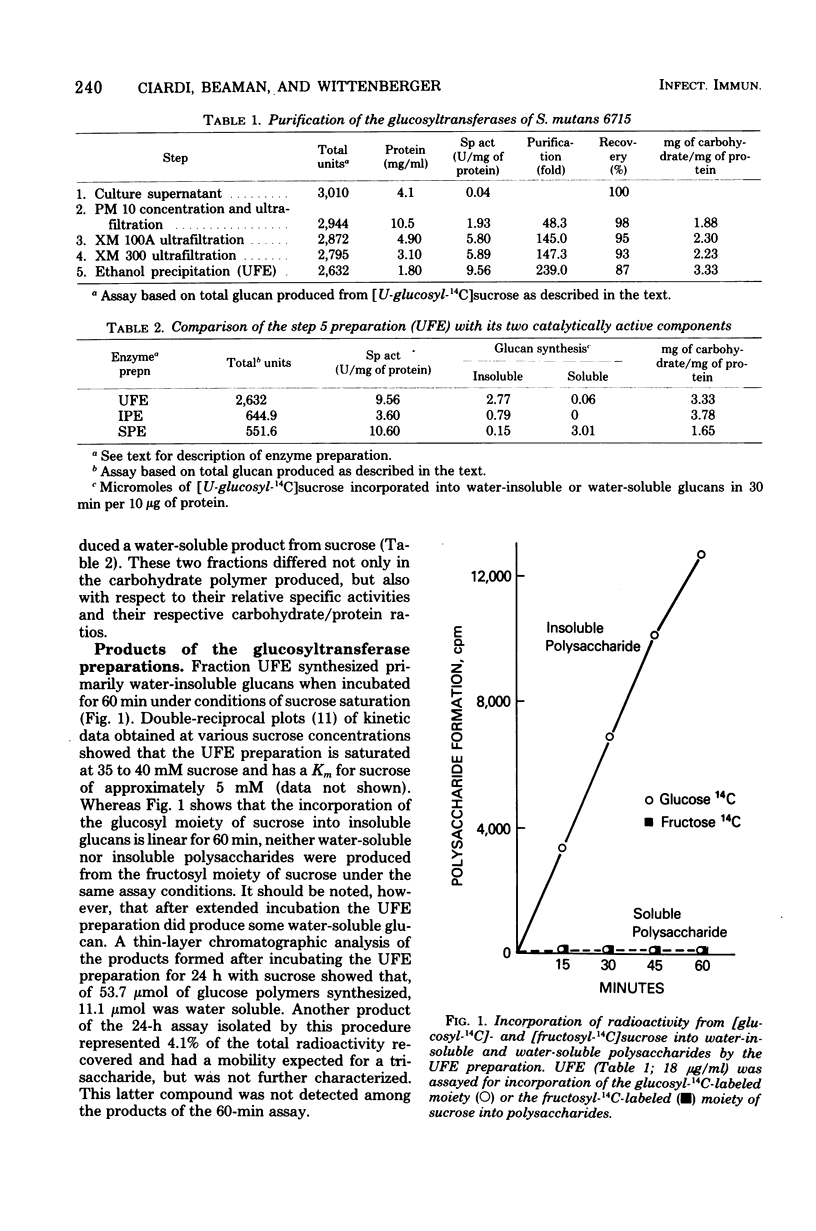
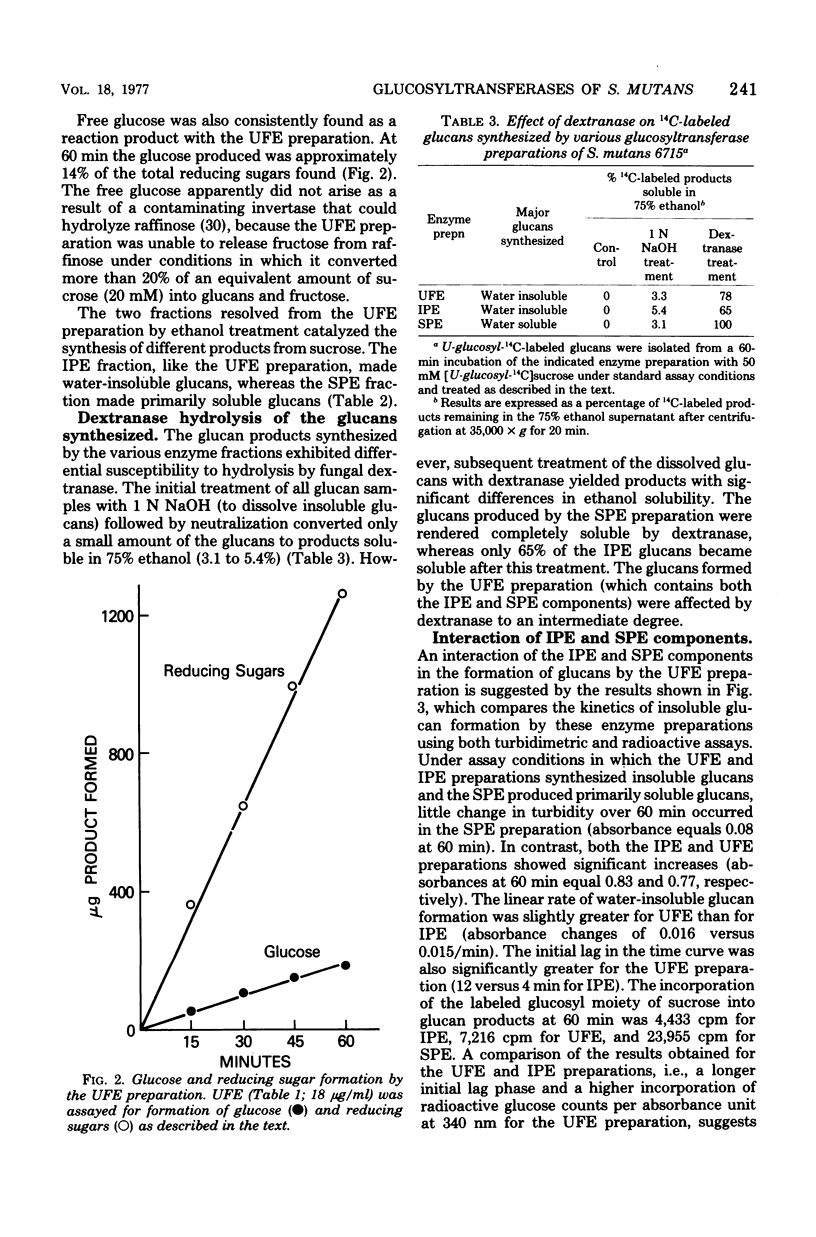
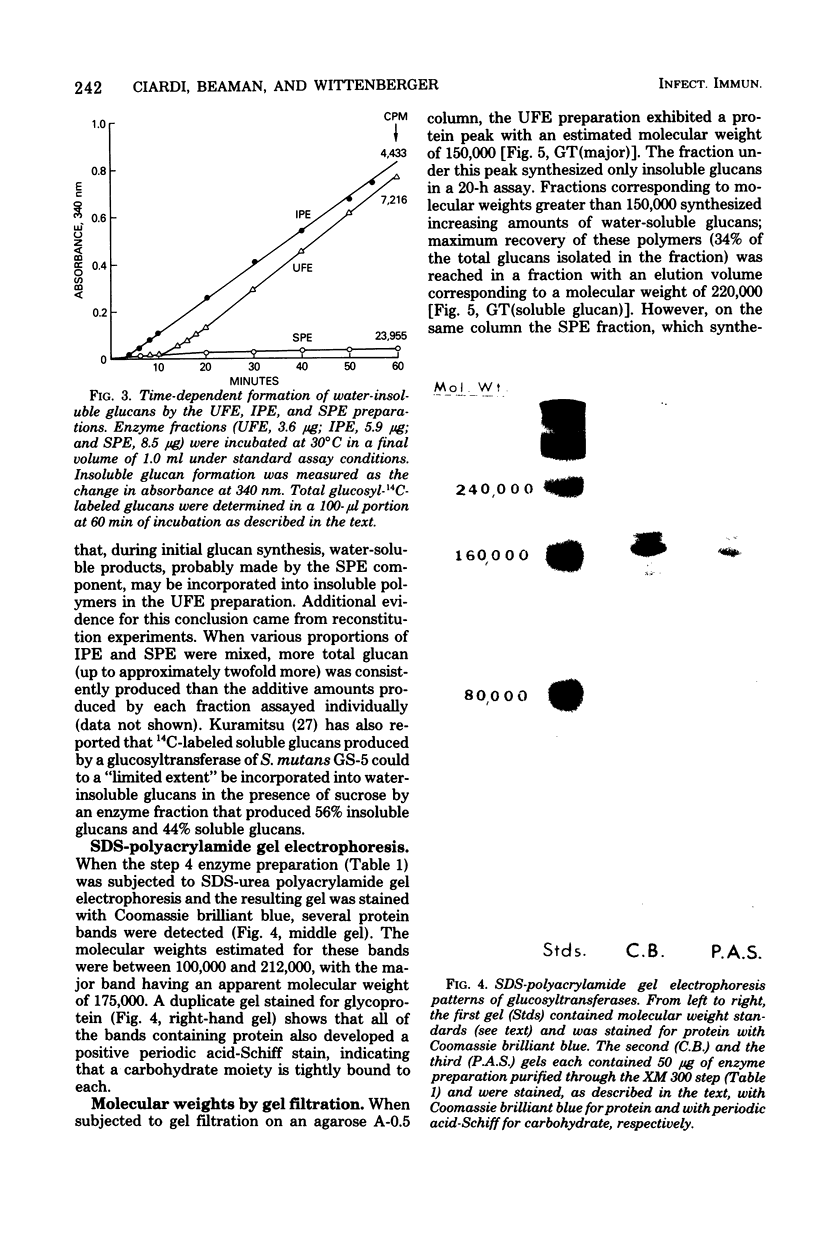
The extracellular glucosyltransferase produced by Streptococcus mutans 6715 was purified from culture supernatant fluids to a specific activity of 9.6 IU/mg of protein, with an overall recovery of 87%. The purified enzyme preparation, designated unfractionated enzyme (UFE), synthesized only water-insoluble glucans from sucrose during the initial stages of the reaction, although some water-soluble polymers accumulated after extended periods of incubation. It was free from measurable fructosyltransferase activity. The UFE preparation was resolved into two different catalytically active components by ethanol fractionation. One fraction (designated insoluble product enzyme [IPE]) synthesized water-insoluble glucans, whereas the other (designated soluble product enzyme [SPE]) produced primarily water-soluble glucans. The difference between the insoluble glucans made by the UFE preparation and those made by the IPE fraction appeared to be due to interaction of the SPE and IPE components in the UFE preparation. Addition of commercial dextrans or enzymatically prepared glucans to the glucosyltransferase assay altered the amounts of soluble and insoluble glucans synthesized by the UFE preparation. The molecular weights of the major enzymatically active proteins producing insoluble and soluble glucans were estimated by gel filtration chromatography to be 150,000 and 175,000, respectively.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen W. H. Effects of dextranase on cariogenic and non-cariogenic dextrans. Br Dent J. 1968 Apr 16;124(8):347–349. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chaiet L., Kempf A. J., Harman R., Kaczka E., Weston R., Nollstadt K., Wolf F. J. Isolation of a pure dextranase from Penicillium funiculosum. Appl Microbiol. 1970 Sep;20(3):421–426. doi: 10.1128/am.20.3.421-426.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Lewis M. S., Folk J. E. Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem. 1974 Feb 10;249(3):940–950. [PubMed] [Google Scholar]
- Ciardi J. E., Cimino F., Stadtman E. R. Multiple forms of glutamine synthetase. Hybrid formation by association of adenylylated and unadenylylated subunits. Biochemistry. 1973 Oct 23;12(22):4321–4330. doi: 10.1021/bi00746a004. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Isolation and purification of Flavobacterium alpha-1,3-glucanase-hydrolyzing, insoluble, sticky glucan of Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1489–1501. doi: 10.1128/jb.124.3.1489-1501.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN A., WEIL R. M., STERN K. G. On the mechanism of dextran formation; chromatographic studies with C14-labeled sugars. J Biol Chem. 1955 Dec;217(2):977–985. [PubMed] [Google Scholar]
- Genco R. J., Evans R. T., Taubman M. A. Specificity of antibodies to Streptococcus mutans; significance in inhibition of adherence. Adv Exp Med Biol. 1974;45(0):327–336. doi: 10.1007/978-1-4613-4550-3_39. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELY W. B. Dextran: structure and synthesis. Adv Carbohydr Chem. 1960;15:341–369. doi: 10.1016/s0096-5332(08)60191-5. [DOI] [PubMed] [Google Scholar]
- Olson G. A., Guggenheim B., Small P. A., Jr Antibody-mediated inhibition of dextran-sucrose-induced agglutination of Streptococcus mutans. Infect Immun. 1974 Feb;9(2):273–278. doi: 10.1128/iai.9.2.273-278.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Schachtele C. F. Evaluation of dextranase production by the cariogenic bacterium Streptococcus mutans. Infect Immun. 1974 Feb;9(2):467–469. doi: 10.1128/iai.9.2.467-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J. Some properties of a dextranglucosidase isolated from oral streptococci and its use in studies on dextran synthesis. J Dent Res. 1972 Mar-Apr;51(2):409–414. doi: 10.1177/00220345720510022901. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]