Abstract
This study used event-related potentials to assess effects of low-level prenatal lead exposure on auditory recognition memory in 2-month-old infants. Infants were divided into four groups according to cord-blood lead concentration: 1) < 2.00 μg/dL, 2) 2.00-2.99 μg/dL, 3) 3.0-3.7 μg/dL, and 4) ≥ 3.7 μg/dL. The first group showed the normally expected differences in P2, P750, and LSW amplitudes elicited by mothers’ and strangers’ voices. These differences were not observed for one or more ERP components in the other groups. Thus, there was electrophysiological evidence of poorer auditory recognition memory at 2 months with cord-blood lead ≥ 2.00 μg/dL.
Keywords: lead (Pb), auditory recognition memory, infant, event-related potentials (ERPs)
Both prenatal and postnatal exposures to lead (Pb) adversely affect neurocognitive and behavioral development in infants and young children (Boucher et al., 2012; Boucher et al., 2009; Chiodo, Jacobson, & Jacobson, 2004; Rothenberg, Poblano, & Schnaas, 2000), with substantial individual, economic, and social costs (Attina & Trasande, 2013; Grosse, Matte, Schwartz, & Jackson, 2002; Reyes, 2007). Despite of efforts to reduce Pb contamination, a large population is still at risk for excessive exposure irrespective of national development. For example, today children from at least four million US households are being exposed to Pb at levels that can harm their development.
The US CDC considered 10 μg/dL as the intervention threshold for Pb exposure until recently (Roper, Houk, Falk, & Binder, 1991). Many studies have found adverse effects of blood Pb level lower than 10 μg/dL (Chiodo et al., 2004; Gump et al., 2008; Lanphear, Dietrich, Auinger, & Cox, 2000; Plusquellec et al., 2007; Shen et al., 1998). These findings led to a rethinking of the lower limits of “safe” lead exposure. The CDC Advisory Committee on Childhood Lead Poisoning Prevention stated in a 2012 report “no level of Pb appears to be safe” for children (Kuehn, 2012). As a result, the CDC redefined the action level of blood Pb to 5 μg/dL (Wheeler & Brown, 2013). This change led to more than 500,000 US children aged 1-5 years at risk for Pb exposure higher than this reference level.
Pb exposure in pregnancy is especially worrisome, because Pb can be transferred from mother to fetus through the placenta (Gardella, 2001). Since the fetus develops at a rapid pace, even minor prenatal Pb exposure might disrupt development (Jedrychowski et al., 2009; Shen et al., 1998). Previous studies have indicated that low-level prenatal Pb exposure can adversely affect sensory and neurocognitive development in infants and young children (Ethier et al., 2012; Gump et al., 2008; Hu et al., 2006).
Memory is one such cognitive function. In adults, chronic Pb exposure affected the recall of previously learned verbal material (Bleecker et al., 2005). In school-aged children, Pb exposure affected short-term memory (Lanphear et al., 2000). However, the impact of low-level prenatal Pb exposure on memory functions in infancy is not clear, due in part to the difficulty of assessing specific neurocognitive function in young infants. Recognition memory, the capability to retrieve previously encountered events or material, steadily improves during the first year of life (Nelson & Collins, 1992; Rose, 1983). The hippocampus and related structures are important neural substrates for recognition memory (McGaugh, Cahill, & Roozendaal, 1996; Nelson, 1995; Siddappa et al., 2004), and animal models show that both prenatal and postnatal Pb exposures have detrimental effects on the developing hippocampus (Baranowska-Bosiacka et al., 2013; Schneider, Lee, Anderson, Zuck, & Lidsky, 2001). Thus, infants’ recognition memory might be vulnerable to prenatal Pb exposure. The goal of this study was to assess the effects of low-level prenatal Pb exposure on infant recognition memory at two months postnatal age.
Assessing memory in very young infants is challenging, because they cannot make overt behavioral responses in the experimental paradigms used with older children and adults. As a result, most previous studies asked parents/guardians to evaluate their babies’ memory development. The subjectivity inherent in this method might reduce researchers’ ability to detect relationships between Pb exposure and neurocognitive functioning (Plusquellec et al., 2007). This study used event-related potentials (ERPs), a sophisticated but non-invasive method of measuring brain activity, to assess recognition memory. ERPs do not require the participants to make overt responses, and the high time resolution of ERPs allows tracking of different cognitive processes involved in recognition memory, such as stimuli discrimination and memory updating.
To consider the effect of prenatal Pb exposure, the authors applied an auditory recognition memory task in which voices of the mother and a stranger are presented to infants while they are awake (deRegnier, Nelson, Thomas, Wewerka, & Georgieff, 2000). There are no differences in the acoustic properties of mother's and stranger's voices in this task. Thus, differential electrophysiological responses elicited by the two voice stimuli suggest that an infant remembers the familiar mother's voice and discriminates it from the novel stranger's voice (Mai et al., 2012). According to previous studies (Mai et al., 2012; Siddappa et al., 2004), three ERPs components are elicited in young infants in this task: P2, P750, and late slow wave (LSW). P2 is thought to measure the discrimination between voices or an attention-modulated process. P750 is considered to reflect processing of the second syllable in a 2-syllable word. LSW is widely used as an index of memory updating. In this auditory recognition memory task, the normal developmental patterns indicating that the infant recognizes the mother's voice are indicated by differences in the amplitudes of P2, P750 and LSW elicited by the mother's vs. a stranger's voice. The authors predicted that low-level prenatal Pb exposure would contribute to a delay in the development of recognition memory in infants, as evidenced by a lack of the expected differences in amplitude between mother and stranger conditions for P2, P750, or LSW.
Method
Participants
The participants in this study were recruited in a longitudinal study. Enrollment occurred between December 2008 and November 2011 in Fuyang County, a rural area near southeastern China. Pregnant women receiving prenatal care at Fuyang Maternal and Children's Health Care Hospital were randomly screened at the routine visit at 36-37 weeks gestation. Those with normal uncomplicated pregnancies were invited to participate in the study. Based on chart review and maternal interview, the following entrance criteria were confirmed: singleton birth with gestational age ≥ 37 weeks, birth weight ≥ 2500 g, 5-minute Apgar score ≥ 7, no use of antibiotics (an indirect measure of confirmed or suspected infection), no placental abruption or uterine rupture, no birth injury or congenital deficits, no hemolytic or metabolic diseases, and normal hearing ability.
Experimenters recorded the ERPs data in the auditory recognition memory task for 254 infants at 2 months. Of these 107 infants qualified for final analysis. Ninety-two infants were excluded for inadequate ERPs data (more than 30% data were removed). Because iron deficiency may also affect the hippocampus-based recognition memory (Burden et al., 2007; Congdon et al., 2012; Lukowski et al., 2010), we excluded 53 infants with evidence of prenatal iron deficiency (ferritin concentration lower than 75μg/l (Amin et al., 2010; Tamura et al., 2002) or ZPP higher than 118 μmol/mol (McLimore et al., 2013)). Two infants who did not have cord blood Pb measurements were also excluded. The study was carried out in the Children's Hospital of Zhejiang University in China. The study was approved by the Institutional Review Board of the University of Michigan and the Children's Hospital of Zhejiang University. Parents provided signed informed consent.
Lead (Pb) exposure
Cord blood samples for Pb were stored at −20° C until analyzed. Pb was analyzed in whole blood in batch by the PE700 method of graphite furnace atomic absorption spectroscopy (Perkin-Elmer Corp., AAnalyst 700, Bodenseewerk, D-88647 Uberlingen). A new device was used after the second cohort in this longitudinal study without change in analysis method (analytikjeana Corp., ZEEnit 700P). The device was calibrated with reference specimens in the beginning and the end of an analysis for each batch of samples. Accuracy and precision of blood Pb analyses were tested (a) at the Center of Clinical Laboratory, Health Ministry of China in its own interlaboratory calibration program, and (b) in the internal quality - control program of the Institute of Occupational Health and Poison Control, the Center for Disease Control and Prevention of China. Using quartiles, we divided infants into four groups according to their Pb levels: 1) < 2.00 μg/dL, n = 21 (12 males), 2) 2.00-2.99 μg/dL, n = 31 (14 males), 3) 3.0-3.7 μg/dL, n = 26 (12 males), and 4) ≥ 3.7 μg/dL, n = 29 (17 males). The Pb level of seven infants in the fourth group was lower than10 μg/dL but higher than 5.00 μg/dL, the new CDC reference level.
Stimuli and Procedure
In the auditory recognition memory task, the stimuli were composed of the mother's and a stranger's voices, the intensity of which were 65 dB. The stranger's voice was the voice of the previously tested mother and thus varied for each infant. The content of the stimuli was the digitized Chinese words “baobao,” meaning “baby,” lasting 750 msec. The two syllables of the Chinese words used as stimuli have different tones. There were 100 trials for each stimulus. Stimuli were randomly presented with the restriction that the same stimulus was not presented in three or more consecutive trials. The interval between trials was randomized between 2250 and 3250 ms. The whole recording session lasted around 12 min.
Testing occurred at the Children's Hospital of Zhejiang University in an electrically shielded quiet room. Infants were seated on the mother's lap, and mothers were instructed to stay as still as possible. The stimuli were presented through two loudspeakers when infants were in a behavioral state of quite alertness. To reduce infant movement, quiet visual stimuli were presented based on a previous study (Dawson, Klinger, Panagiotides, Hill, & Spieker, 1992).
Confounding variables
Potential confounding variables are presented in Table 1. They were considered as covariates in the final data analysis if the p values for the differences between Pb groups were lower than 0.1.
Table 1.
Infant characteristics and family background by group
| Pb group (quartile) (n) | Group 1 Pb < 2.00 μg/dL (21) | Group 2 Pb = 2.00-2.99 μg/dL (31) | Group 3 Pb= 3.00-3.70 μg/dL (26) | Group 4 Pb ≥ 3.70 μg/dL (29) |
|---|---|---|---|---|
| Infant characteristics | ||||
| Gender (% male, n) | 57.1 (12) | 45.2 (14) | 46.2 (12) | 58.6 (17) |
| Deliver type (% vaginal, n) | 33.3 (7) | 35.5 (11) | 26.9 (7) | 24.1 (7) |
| Gestation age a | 39.39 (1.14) | 39.82 (0.96) | 39.23 (0.96) | 39.49 (0.77) |
| Birth weight (kg) a | 3.47 (0.46) | 3.40 (0.35) | 3.35 (0.35) | 3.42 (0.42) |
| Age (days) a | 42.19 (2.52) | 42.58 (1.59) | 43.77 (2.37) | 43.03 (2.18) |
| Weight-for-age z score a | 0.65 (0.74) | 0.50 (0.79) | 0.54 (0.81) | 0.55 (0.88) |
| Head circumference-for-age z score a | 0.71 (0.98) | 0.19 (0.81) | 0.06 (1.04) | 0.05 (0.88) |
| Valid trials for mother condition a | 51.95 (14.41) | 54.74 (15.59) | 57.96 (20.38) | 55.83 (13.69) |
| Valid trials for stranger condition a | 53.19 (13.60) | 57.45 (16.98) | 55.65 (19.54) | 56.76 (14.29) |
| Family background | ||||
| Mother's age (years) a | 26.00 (3.82) | 27.39 (3.52) | 26.27 (3.07) | 27.00 (3.63) |
| Number in family a | 5.19 (1.03) | 5.03 (1.52) | 5.56 (1.53) | 5.24 (1.06) |
| First born (%, n) | 85.7 (18) | 71.0 (22) | 76.9 (20) | 77.6 (83) |
| Father in home (%, n) | 100 (21) | 93.5 (29) | 92.3 (24) | 93.1 (27) |
| Grandparents in home (%, n) | 90.5 (19) | 83.9 (26) | 80.8 (21) | 86.2 (25) |
| Parents' education (% >high school, n) | 76.2 (16) | 71 (22) | 64 (16) | 58.6 (17) |
| Father smokes (%, n) | 40 (8) | 48.3 (14) | 73.9 (17) | 51.9 (14) |
Note.
Mean values and standard deviation were presented for these variables.
ERPs recording and analysis
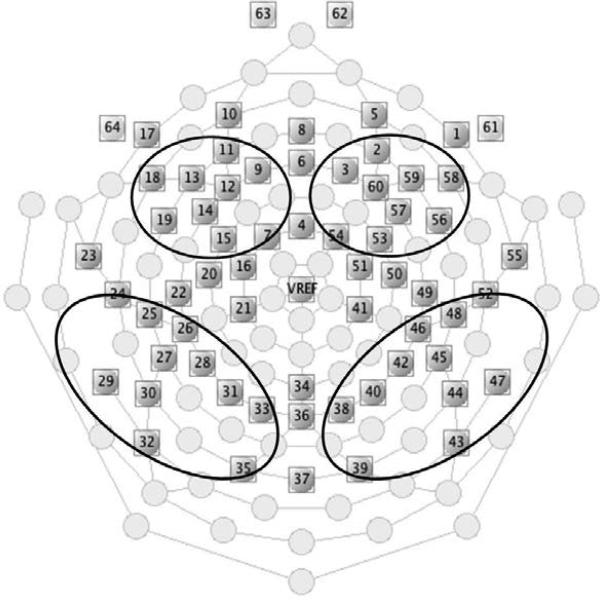
A 64-channel HydroCel Geodesic Sensor net was used to record the electroencephalogram (EEG) data (Electrical Geodesics Inc., Eugene, OR), see Figure 1. However, four eye channels (61, 62, 63, and 64) were not used, leading to a 60-channel recording. The recording in every channel was referenced to the vertex electrode. The EEG signal was sampled at 500 Hz/channel and filtered online with a 0.1-100 Hz bandpass. The impedance for each channel was kept below 50 kΩ across the testing.
Figure 1.
Layout of the EGI 64-channel sensor net. The marked clusters were the four regions of interest defined for P2, P750, and LSW.
The EEG data was processed using Net Station 4.3 (Eugene, OR). A 30-Hz low-pass filter was performed, and all trials were segmented 200 ms before and 2000 ms after the onset of stimuli. The EEG data was then baseline corrected to the average voltage during the 200 ms prior to stimuli onset. The remaining four eye channels (1, 5, 10, and 17) were then used for identifying ocular artifacts in the segmented data. Additionally, each epoch was inspected for artifact caused by motion or poor electrode contact. For each segment data from individual channels were rejected if artifact was detected or the amplitude was higher than 150 μV. The entire trial was excluded if more than nine electrodes were marked as bad or if eye blink, eye movement, or other significant artifacts had been detected. For the remaining trials, spherical spline interpolation was used to construct the rejected channels. Infants who had at least 30 artifact-free trials for each condition were included for further analysis. The number of valid trials was computed separately for mother and stranger conditions (M = 55.3, range: 30-88, SD = 16.1; M = 55.9, range: 30-92, SD = 16.2). There was no difference between mother and stranger conditions in the mean number of valid trials (t (106) = −1.12, p = .26). Additionally, there were no differences between the four Pb groups in the mean number of valid trials for each condition (F (3, 107) = .55, p = .65; F (3, 107) = .31, p = .82). The valid number of trials for each condition and group was presented in Table 1.
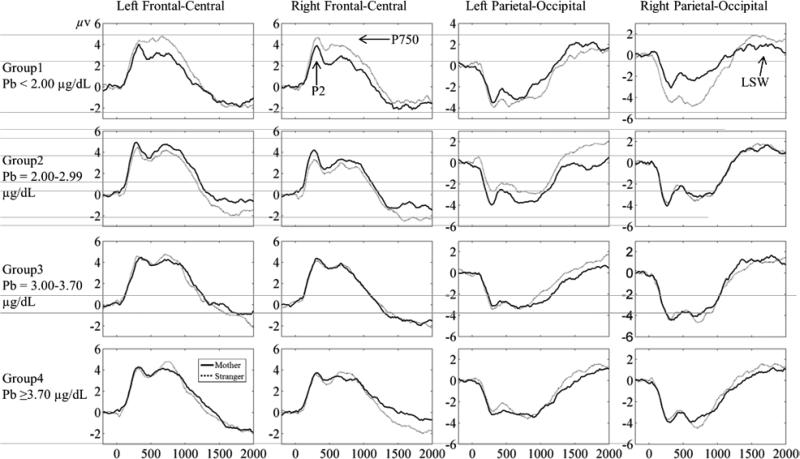
According to previous studies (deRegnier et al., 2000; deRegnier, Wewerka, Georgieff, Mattia, & Nelson, 2002; Mai et al., 2012) and the distribution of the three ERP components in our data, four regions of interest (ROI) were defined for further analysis: left frontal-central (including channel 7, 9, 11, 12, 13, 14, 15, 18, and 19), right frontal-central (including channel 2, 3, 53, 54, 56, 57, 58, 59, and 60), left parietal-occipital (including channel 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, and 35), and right parietal-occipital (including channel 38, 39, 40, 42, 43, 44, 45, 46, 47, 48, and 52), see Figure 1. Figure 2 displays the merged ERP waveforms for each ROI and group. P2 and P750 were identified as positive peaks in frontal-central regions and as negative peaks in parietal-occipital regions because of polarity inversion, separately occurring approximately 200 ms and 750 ms after stimulus onset. LSW was identified as positive-going slow wave in front-central regions and negative-going slow wave in parietal-occipital regions, occurring 1500 post stimulus onset. ERPs from frontal-central and parietal-occipital regions established the factor of front-back in subsequent statistical analysis, while ERPs from left and right hemisphere ROIs established the hemisphere factor. The mean of the channels in each region was computed to quantify ERPs. In accord with Mai et al. (2012), the mean amplitude of P2, P750, and LSW was separately computed between 150-400 ms, 500-1000 ms, and 1500-2000 ms after the onset of stimuli.
Figure 2.
Merged ERP waveforms for each region of interest and group
Statistical analysis
For P2, P750 and LSW, mean amplitudes were used in repeated ANOVA analyses with condition (mother, stranger), front-back (frontal-central, parietal-occipital), and hemisphere (left, right) as within-subject factors and Pb group as between-subject factor. Greenhouse-Geisser correction was applied if the assumption of sphericity was violated. Follow-up ANOVA analyses were carried out for significant interactions.
Results
Sample characteristics
The characteristics of the infants and their families are presented in Table 1 by Pb group. There was no group difference in these variables. Head circumference and age were included as covariates in the final analysis of P2, P750, and LSW.
P2
There was a main effect of front-back for P2 (F (1,101) = 248.67, p = .000, ηp2= .71). This expected result indicated polarity inversion across the scalp. There was also a main effect of hemisphere (F (1,101) = 10.16, p = .002, ηp2 = .09). The amplitude in the right hemisphere was more negative compared with the left hemisphere. There was a three-way interaction involving Pb group: Condition × Front-back × Group (F (3, 101) = 3.82, p = .012, ηp2 = 0.10). Further ANOVA analyses were then carried out to see if there was interaction between condition and front-back in each Pb group. There were two interactions in group 1: Condition × Front-back (F (1, 18) = 5.98, p = .025, ηp2 = .25), Condition × Front-back × Hemisphere (F (1, 18) = 5.75, p = .028, ηp2 = .24). The main effect of condition was significant in parietal-occipital locations (F (1, 18) = 6.81, p = .018, ηp2 = .27). The result indicated that P2 amplitude in the stranger condition was more negative than that of the mother condition in parietal-occipital location.
An interaction between condition and front-back was also found in group 2 (F (1, 28) = 4.94, p = .035, ηp2 = .15). There was a main effect of condition in the frontal-central location (F (1, 28) = 4.67, p = .039, ηp2 = .14). This effect was also significant in the parietal-occipital location (F (1, 28) = 4.61, p = .041, ηp2 = .14). These results indicate that P2 amplitude in mother condition was more positive than that of stranger condition in the frontal-central location for the infants in group 2, but P2 in mother condition tended to be more negative than that of stranger condition in the parietal-occipital location. The direction of the condition differences for the infants in group 2 was in opposite of the infants in group 1. The interaction between condition and front-back was not found in groups 3 and 4.
P750
There was a main effect of front-back for P750, reflecting the polarity inversion across the scalp (F (1, 101) = 392.04, p = .000, ηp2 = .80). There was also a main effect of hemisphere (F (1, 101) = 8.77, p = .004, ηp2 = .08), indicating that the amplitude in the right hemisphere was more negative than the amplitude in the left hemisphere. There was also an interaction between front-back and hemisphere (F (1, 101) = 6.03, p = .016, ηp2 = .06). The amplitude was more positive in the left vs. the right hemisphere in the frontal-central location (F (1, 101) = 15.46, p = .000, ηp2= .13), but such an effect was not found in the parietal-occipital location
There was a three-way interaction involving Pb group: Condition × Front-back × Group (F (3, 101) = 4.79, p = .004, ηp2 = .13). Separate ANOVA analyses indicated that the interaction between condition and front-back was significant only in group 1 (F (1, 18) = 9.83, p = .006, ηp2 = .35). To break down this two-way interaction, frontal-central and parietal-occipital locations were analyzed separately. The P750 amplitude for the stranger condition was more positive than mother condition in the frontal-central location (F (1, 18) = 7.66, p = .013, ηp2 = .30), while the P750 amplitude for the stranger condition was more negative than mother condition in the parietal-occipital location (F (1, 18) = 10.79, p = .004, ηp2 = .38). The interaction between condition and front-back was not significant in the other Pb groups.
LSW
There was a main effect of front-back for LSW, indicating the polarity inversion across the scalp (F (1, 101) = 51.39, p = .000, ηp2 = .34). There was an interaction between condition and front-back (F (1, 101) = 6.85, p = .010, ηp2 = .06). LSW amplitude was more negative in the stranger vs. mother condition in the frontal-central location (F (1, 101) = 5.89, p =.017, ηp2 = .06). In contrast, the LSW amplitude was more positive in the stranger vs. mother condition in the parietal-occipital location (F (1, 101) = 6.60, p = .012, ηp2 = .06).
There was also a three-way interaction involving Pb group: Front-back × Hemisphere × Group (F (1, 101) = 3.39, p = .021, ηp2 = .09). To understand this interaction, further analysis was carried within each group to test if there was an interaction between front-back and hemisphere. The results revealed such an interaction in groups 2 and 3 (F (1, 28) = 6.73, p = .015, ηp2= .19; F (1, 23) = 6.57, p = .017, ηp2 = .22). Further analysis was then carried out to break down this two-way interaction for groups 2 and 3. The results indicated that the main effect of front-back was significant in the right hemisphere for groups 2 and 3 (F (1, 28) = 14.97, p = .001, ηp2 = .35; F (1, 28) = 11.00, p = .003, ηp2 = .32). This main effect was not found in the left hemisphere for both groups.
Discussion
This study investigated the effects of low-level prenatal Pb exposure on auditory recognition memory in 2-month-old infants. Only the infants with the lowest Pb concentration in cord blood (< 2.00 μg/dL) showed all the expected differences in the amplitudes of P2, P750, and LSW between mother and stranger conditions. In contrast, the normal condition differences observed in group 1 were not observed for at least one ERP component for the infants in the other three groups with Pb concentration in cord blood higher than 2.00 μg/dL. Overall, these findings suggest that even very low prenatal Pb exposure adversely affects the development of auditory recognition memory in 2-month-old infants.
As to P2, infants with the lowest Pb concentration at birth had amplitude for stranger condition that was more negative than mother condition in the parietal-occipital location. In contrast, for the infants with cord Pb between 2.00 and 2.99 μg/dL, the P2 amplitude of the stranger condition was less positive in the frontal-central location and less negative in the parietal-occipital location compared with the mother condition. Condition differences were not found in the groups with cord Pb higher than 2.99 μg/dL. As P2 has been suggested to measure voice discrimination (deRegnier et al., 2000; Mai et al., 2012), the results indicate that only those infants with prenatal Pb exposure lower than 2.99 μg/dL showed electrophysiological evidence of detecting the difference between mother's and stranger's voices at 2 months. P2 has been also suggested to measure attention-modulated process (García-Larrea, Lukaszewicz, & Mauguiére, 1992; Mai et al., 2012). Therefore, our findings might also indicate that those infants with cord Pb lower than 2.99 μg/dL were more likely to use different amounts of attention resources in processing familiar and novel voices in the early phase of cognitive processing. Consistent with this explanation, previous studies have found that prenatal Pb exposure exerted detrimental effects on postnatal attention development (Boucher et al., 2009; Plusquellec et al., 2007). It is worth noting that infants in groups 1 and 2 showed the opposite pattern in P2 amplitude differences between mother and stranger conditions. Infants in group 1 invested more neural resources to process stranger vs. mother voices, whereas infants in group 2 used more neural resources to process mother vs. stranger voices. We can infer that the infants in group 1 showed a preference for novel stimuli, whereas infants in group 2 had a bias toward familiar stimuli. Previous studies have shown that infants gradually shift their preference for familiarity to novelty during the first year of life (Fantz, 1964; Perone & Spencer, 2013; Rose, Feldman, & Jankowski, 2004). Thus, the above difference between groups 1 and 2 might suggest a delay in the familiarity-novelty shift in group 2.
With respect to P750, only infants with cord Pb lower than 2.00 μg/dL had amplitude for the stranger condition that was more positive in the frontal-central location and more negative in the parietal-occipital location compared with the mother condition. As P750 is thought to measure the processing of the second syllable of a two-syllable word (deRegnier et al., 2000; Mai et al., 2012), the above results might indicate that infants with cord Pb < 2.00 μg/dL at birth were more likely to invest more brain resources in processing the second syllable of “baby” in Chinese as spoken by strangers vs. mothers. This finding adds support to our interpretation that only the infants with the lowest prenatal Pb exposure showed a preference for unfamiliarity. Moreover, the P2 and P750 findings together might also suggest that only the infants in group 1 were able to encode novel stimuli during the early sensory processing stage.
For the LSW, the amplitude of the stranger condition was more negative in the frontal-central location and more positive in the parietal-occipital location relative to the mother condition, regardless of Pb group. Negative-going LSW in the frontal-central location and positive-going LSW in the parietal-occipital location are thought to reflect novelty detecting and memory updating, respectively (Mai et al., 2012). However, as discussed above, only the infants in group 1 showed condition differences in both P2 and P750 amplitude. Therefore, it is speculated that although all infants in this study detected the novelty of the stranger's voice, the infants in group 1 encoded this voice more deeply than the infants in the other groups. Additionally, for the infants in groups 2 and 3, a hemisphere difference was involved in the main effect of front-back. This finding is hard to interpret due to the paucity of previous neural studies about the adverse effect of Pb in infants, but the authors speculate that different levels of prenatal Pb exposure might affect the interaction between the two hemispheres of the brain in different ways.
The LSW findings indicate that all Pb groups in this study were able to detect the novelty of an unfamiliar voice and update their memories of the stimuli. However, the P2 and P750 findings suggest that only infants with the lowest Pb exposure showed evidence of investing more brain resources to process the novel vs. familiar voice. These results are interpreted as evidence that the infants in this group showed stronger novelty preference than the infants with cord Pb ≥ 2.00 μg/dL. Perone and Spencer (2013) proposed that novelty preference provides evidence that a robust memory for the familiar stimuli has been formed and that the infants have started to learn new items. Accordingly, the results imply that those infants with cord Pb < 2.00 μg/dL had built a more robust memory of their mother's voice and had started encoding the information for a novel voice than the other infants. Thus, recognition memory appears to be more mature for infants with prenatal Pb exposure lower than 2.00 μg/dL compared the ones with higher exposure.
There are two promising mechanisms for the adverse effect of prenatal Pb exposure on auditory recognition memory. Prenatal and postnatal Pb exposures disrupt the developing hippocampus in animals models (Baranowska-Bosiacka et al., 2013; Schneider et al., 2001), and the hippocampus plays an important role in the neural circuits related to recognition memory (McGaugh et al., 1996; Nelson, 1995; Siddappa et al., 2004). Therefore, hippocampal dysfunction might be the neural underpinning for the adverse effect of prenatal Pb exposure on recognition memory in 2-month-old infants. Alternatively, Pb exposure has been found to slow down children's speed of processing and disrupt attention functions in infants and young children (Boucher et al., 2009; Chiodo et al., 2004; Plusquellec et al., 2007). Processing speed and attention are important for recognition memory (Rose et al., 2004). Thus, prenatal Pb exposure may delay the development of recognition memory in infants through detrimental effects on the other cognitive functions, such as the speed of processing and attention.
One limitation of this study was that prenatal Pb exposure level was measured only in umbilical cord blood and not during pregnancy. The timing of Pb exposure during pregnancy is important in later neurocognitive development (Hu et al., 2006). Therefore, future research is needed to determine how Pb exposure at different times during a pregnancy affects the development of auditory recognition memory in young infants. Another limitation is that alerting was not directly observed and recorded during testing. However, testing was conducted only for infants who were in a state of quite alertness.
In summary, using sensitive measures of neural functioning, the authors found that cord Pb higher than 2.00 μg/dL was associated with adverse effects on the development of auditory recognition memory at 2 months. It should be emphasized that virtually all children in this study had cord Pb levels below 5.00 μg/dL, the new CDC reference level. Our results suggest that there are adverse effects of prenatal Pb exposure below this level.
Acknowledgments
This work was supported by grants from the National Institute of Health (R01 ES21465 and P01 HD39386). The authors are grateful to Dr. Raye-Ann deRegnier for help with task design, analysis, and interpretation.
References
- Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. The Journal of Pediatrics. 2010;156(3):377–381. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attina TM, Trasande L. Economic costs of childhood lead exposure in low-and middle-income countries. Environmental Health Perspectives. 2013;121(9):1097–1102. doi: 10.1289/ehp.1206424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska-Bosiacka I, Strużyńska L, Gutowska I, Machalińska A, Kolasa A, Kłos P, Chlubek D. Perinatal exposure to lead induces morphological, ultrastructural and molecular alterations in the hippocampus. Toxicology. 2013;303:187–200. doi: 10.1016/j.tox.2012.10.027. doi: http://dx.doi.org/10.1016/j.tox.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Bleecker ML, Ford DP, Lindgren KN, Hoese VM, Walsh KS, Vaughan CG. Differential effects of lead exposure on components of verbal memory. Occupational and Environmental Medicine. 2005;62(3):181–187. doi: 10.1136/oem.2003.011346. doi: 10.1136/oem.2003.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly É, Jacobson JL. Response inhibition and error monitoring during a visual go/no-go task in inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environmental Health Perspectives. 2012;120:608–615. doi: 10.1289/ehp.1103828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Saint-Amour D, Dewailly É, Ayotte P, Jacobson SW, Bastien CH. The relation of lead neurotoxicity to the event-related potential P3b component in Inuit children from arctic Québec. NeuroToxicology. 2009;30(6):1070–1077. doi: 10.1016/j.neuro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120(2):e336–e345. doi: 10.1542/peds.2006-2525. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicology and Teratology. 2004;26(3):359–371. doi: 10.1016/j.ntt.2004.01.010. doi: http://dx.doi.org/10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, Nelson CA. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 Years. J Pediatr. 2012;160(6):1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Hill D, Spieker S. Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child Dev. 1992;63(3):725–737. doi: 10.2307/1131357. [PubMed] [Google Scholar]
- deRegnier R-A, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137(6):777–784. doi: 10.1067/mpd.2000.109149. doi: http://dx.doi.org/10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- deRegnier R-A, Wewerka S, Georgieff MK, Mattia F, Nelson CA. Influences of postconceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Developmental Psychobiology. 2002;41(3):216–225. doi: 10.1002/dev.10070. doi: 10.1002/dev.10070. [DOI] [PubMed] [Google Scholar]
- Ethier A-A, Muckle G, Bastien C, Dewailly É, Ayotte P, Arfken C, Saint-Amour D. Effects of environmental contaminant exposure on visual brain development: A prospective electrophysiological study in school-aged children. NeuroToxicology. 2012;33(5):1075–1085. doi: 10.1016/j.neuro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Fantz RL. Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science. 1964;146(3644):668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Lukaszewicz A-C, Mauguiére F. Revisiting the oddball paradigm. Non-target vs neutral stimuli and the evaluation of ERP attentional effects. Neuropsychologia. 1992;30(8):723–741. doi: 10.1016/0028-3932(92)90042-k. doi: http://dx.doi.org/10.1016/0028-3932(92)90042-K. [DOI] [PubMed] [Google Scholar]
- Gardella C. Lead exposure in pregnancy. Obstetrical & Gynecological Survey. 2001;56(4):231–238. doi: 10.1097/00006254-200104000-00024. doi: 10.1097/00006254-200104000-00024. [DOI] [PubMed] [Google Scholar]
- Grosse SD, Matte TD, Schwartz J, Jackson RJ. Economic gains resulting from the reduction in children's exposure to lead in the United States. Environmental Health Perspectives. 2002;110(6):563–569. doi: 10.1289/ehp.02110563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Parsons PJ, Granger DA. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environmental Health Perspectives. 2008;116(2):249–255. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, Hernández-Avila M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environmental Health Perspectives. 2006;114(11):1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Lisowska-Miszczyk I. Very low prenatal exposure to lead and mental development of children in infancy and early childhood. Neuroepidemiology. 2009;32(4):270–278. doi: 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Panel advises tougher limits on lead exposure. JAMA. 2012;307(5):445–445. doi: 10.1001/jama.2012.50. doi: 10.1001/jama.2012.50. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 μg/dL in US children and adolescents. Public Health Reports (1974-) 2000;115(6):521–529. doi: 10.1093/phr/115.6.521. doi: 10.2307/4598586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13(2):54–70. doi: 10.1179/147683010X12611460763689. doi: 10.1179/147683010x12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai X, Xu L, Li M, Shao J, Zhao Z, deRegnier R-A, Lozoff B. Auditory recognition memory in 2-month-old infants as assessed by event-related potentials. Dev Neuropsychol. 2012;37(5):400–414. doi: 10.1080/87565641.2011.650807. doi: 10.1080/87565641.2011.650807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: Interaction with other brain-systems. Proceedings of the National Academy of Sciences. 1996;93(24):13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer BA, Kling PJ. Impact of multiple prenatal risk factors on newborn iron status at delivery. J. Pediatr. Hematol. Oncol. 2013;35(6):473–477. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;31(5):723–738. doi: 10.1037/0012-1649.31.5.723. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19(1):105–121. doi: 10.1016/0278-2626(92)90039-o. doi: http://dx.doi.org/10.1016/0278-2626(92)90039-O. [DOI] [PubMed] [Google Scholar]
- Perone S, Spencer JP. Autonomous visual exporation creates developmental change in familiarity and novelty seeking behaviors. Frontiers in Psychology. 2013;4:648. doi: 10.3389/fpsyg.2013.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Jacobson SW, Jacobson JL. The relation of low-level prenatal lead exposure to behavioral indicators of attention in Inuit infants in Arctic Quebec. Neurotoxicology and Teratology. 2007;29(5):527–537. doi: 10.1016/j.ntt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JW. Environmental policy as social policy? The impact of childhood lead exposure on crime. The B.E. Journal of Economic Analysis & Policy. 2007;7(1):1–41. [Google Scholar]
- Roper WL, Houk VN, Falk H, Binder S. Preventing lead poisoning in young children: A statement by the Centers for Disease Control, October 1991 Other Information: See also HRP-0027804 (pp. Medium: X; Size: Pages: (110 p)) 1991.
- Rose SA. Differential rates of visual information processing in full-term and preterm infants. Child Development. 1983;54(5):1189–1198. doi: 10.2307/1129674. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24(1):74–100. doi: 10.1037/0012-1649.39.3.563. doi: http://dx.doi.org/10.1016/j.dr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Rothenberg SJ, Poblano A, Schnaas L. Brainstem auditory evoked response at five years and prenatal and postnatal blood lead. Neurotoxicology and Teratology. 2000;22(4):503–510. doi: 10.1016/s0892-0362(00)00079-9. doi: http://dx.doi.org/10.1016/S0892-0362(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Research. 2001;896(1–2):48–55. doi: 10.1016/s0006-8993(00)03249-2. doi: http://dx.doi.org/10.1016/S0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- Shen X-M, Yan C-H, Guo D, Wu S-M, Li R-Q, Huang H, Tang J-M. Low-level prenatal lead exposure and neurobehavioral development of children in the first year of life: A prospective study in Shanghai. Environmental Research. 1998;79(1):1–8. doi: 10.1006/enrs.1998.3851. doi: http://dx.doi.org/10.1006/enrs.1998.3851. [DOI] [PubMed] [Google Scholar]
- Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55(6):1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. The Journal of Pediatrics. 2002;140(2):165–170. doi: 10.1067/mpd.2002.120688. doi: http://dx.doi.org/10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- Wheeler W, Brown MJ. Blood lead levels in children aged 1-5 years-United States, 1999-2010. MMWR-Morbidity and Mortality Weekly Report. 2013;62(13):245–248. [PMC free article] [PubMed] [Google Scholar]