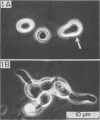
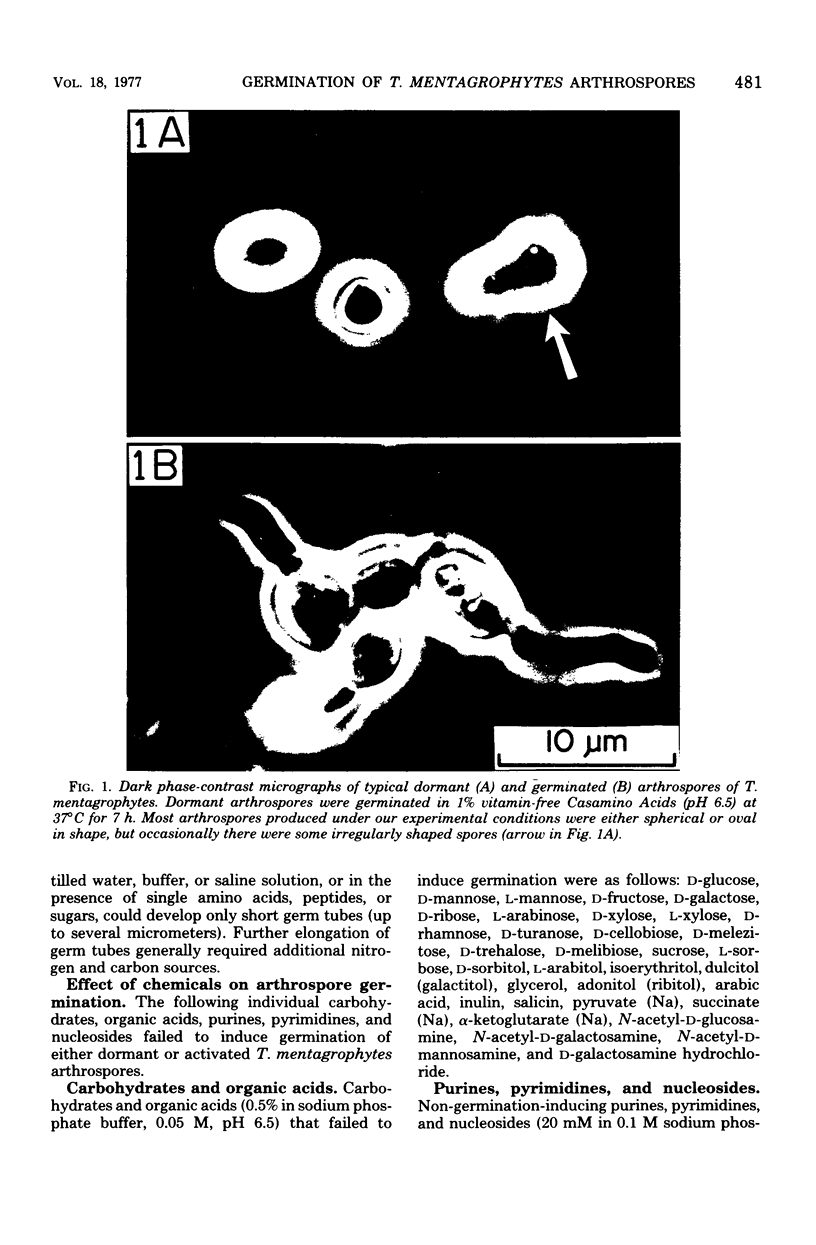
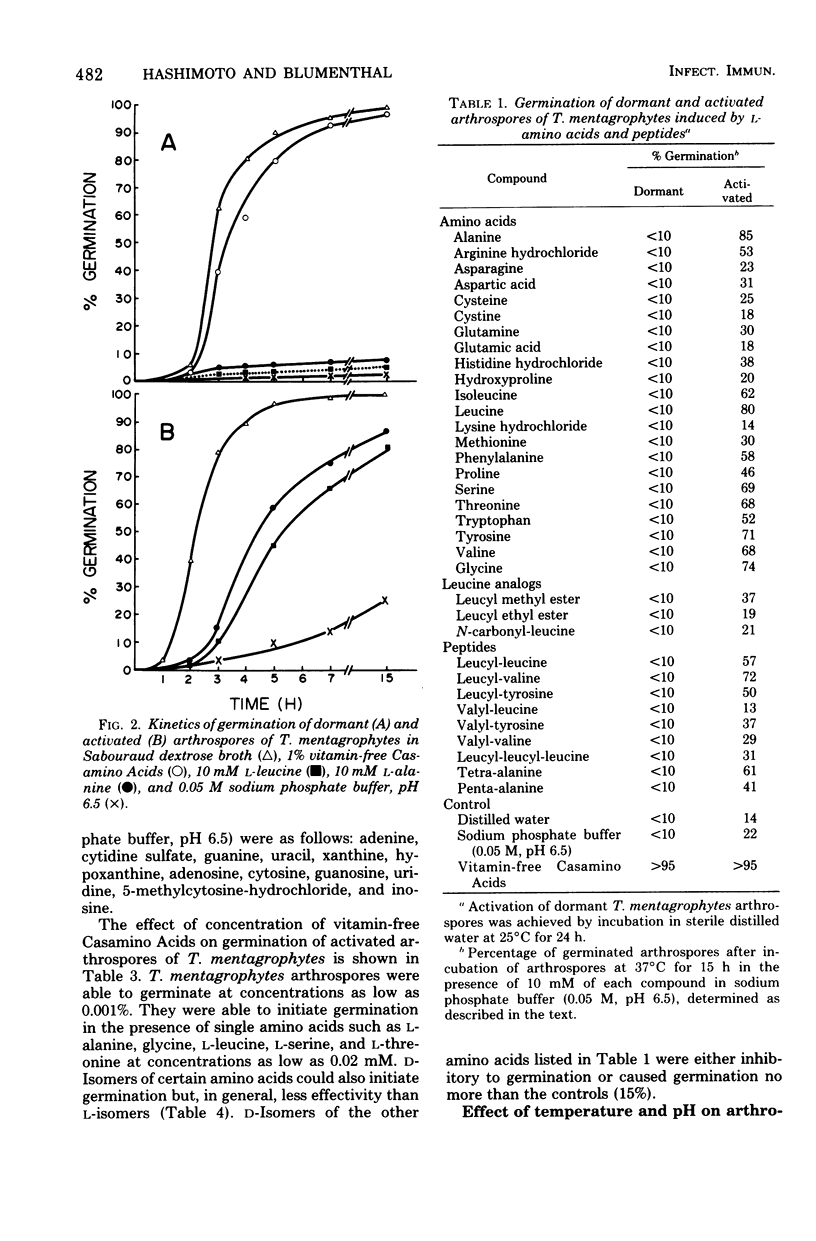
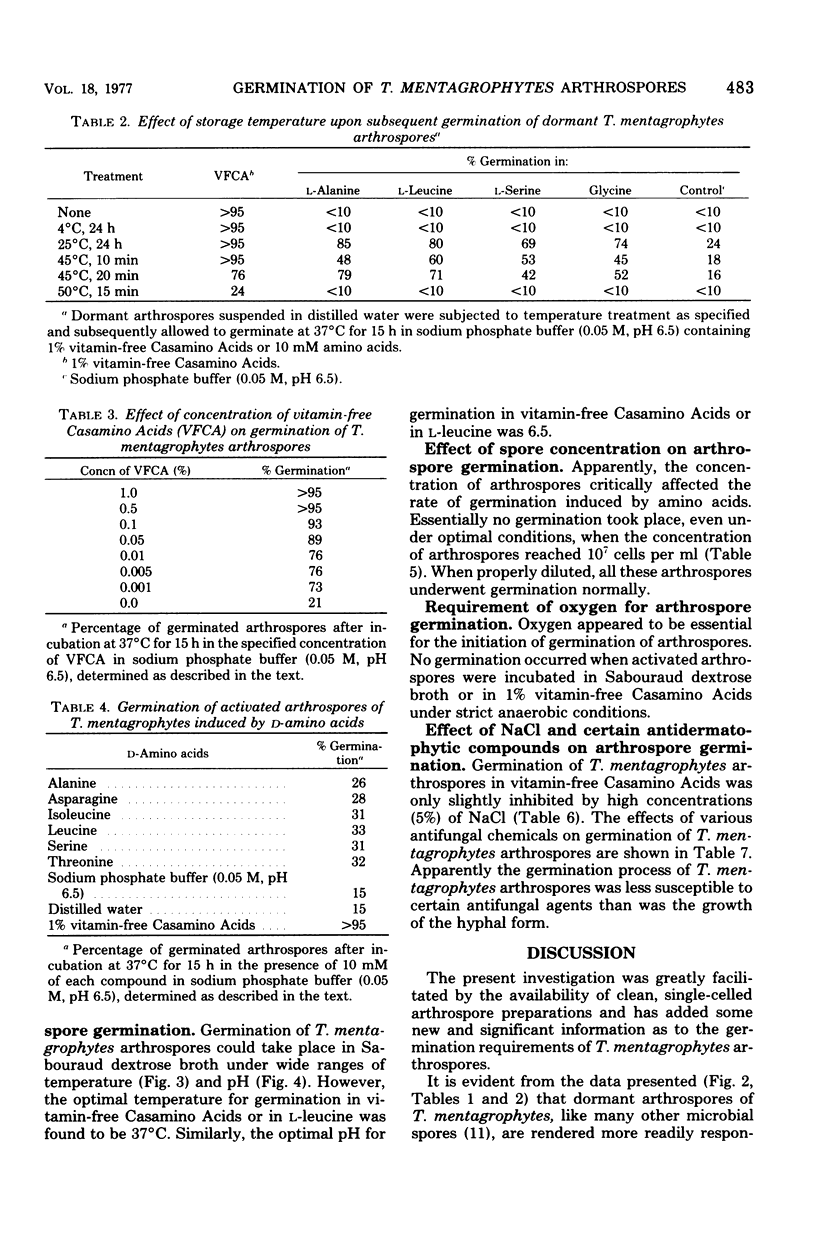
Abstract
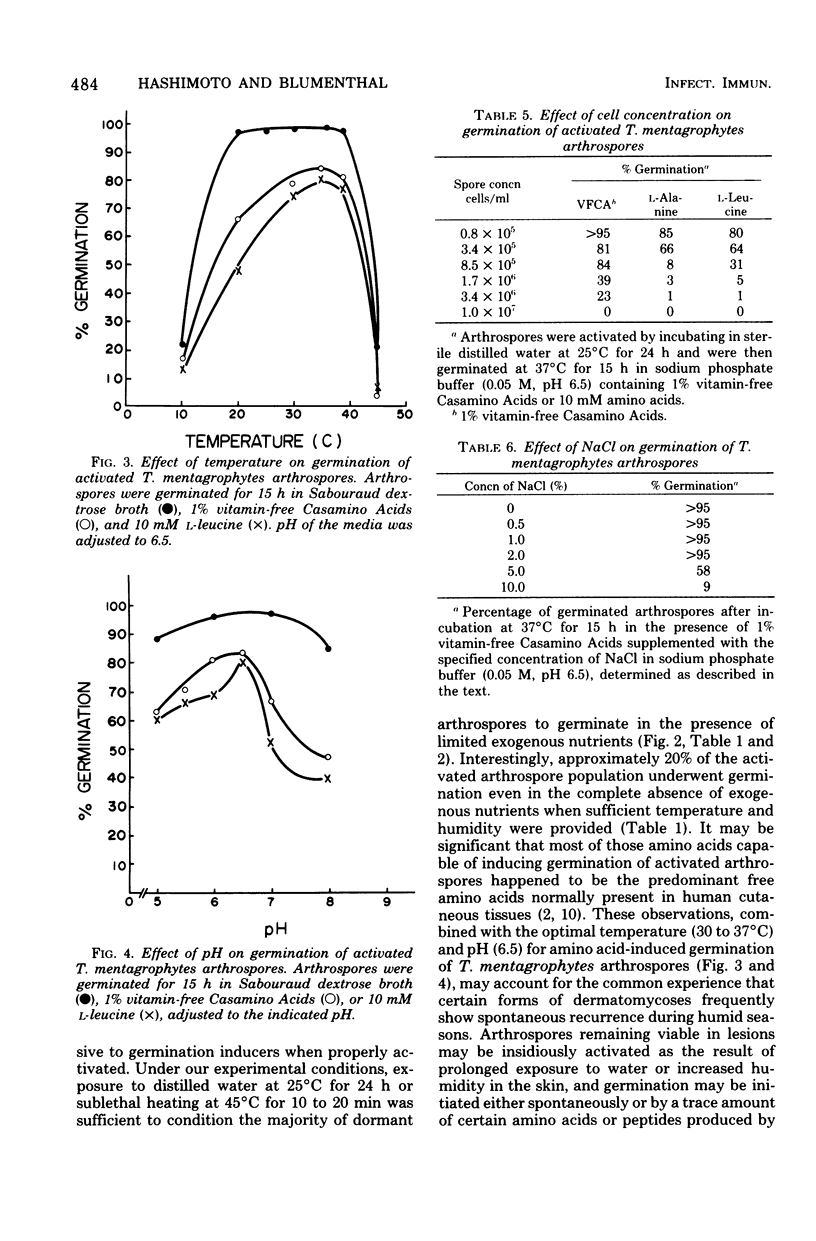
Nutritional and environmental factors affecting germination of Trichophyton mentagrophytes arthrospores were investigated. Germination of dormant arthrospores occurred only in rich complex media such as Sabouraud dextrose broth or vitamin-free Casamino Acids. However, once activated, arthrospores were able to germinate under wide ranges of pH (5.5 to 8.0, optimal 6.5) and temperature (20 to 39 degrees C, optimal 37 degrees C) in the presence of certain single amino acids or oligopeptides known to be present in the human cutaneous tissues. Dormant arthrospores could be activated by incubation in distilled water at 25 degrees C for 24 h or by brief exposure to sublethal doses of heat (45 degrees C for 10 to 20 min). Approximately 20% of activated arthrospores underwent spontaneous germination at 37 degrees C during an additional 18 h of incubation in distilled water. All monosaccharides, purines, pyrimidines, and nucleosides tested failed to induce germination of T. mentagrophytes arthrospores. Germination rate was affected by the concentration of germination inducers as well as that of arthrospores. The germination process of T. mentagrophytes arthrospores was found to be oxygen dependent and was relatively tolerant to NaCl, clotrimazole, cycloheximide, griseofulvin, and tolnaftate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. C., Lee T. H., Buettner-Janusch V. Free amino acids and water soluble peptides in stratum corneum and skin surface film in human beings. Yale J Biol Med. 1966 Feb;38(4):355–373. [PMC free article] [PubMed] [Google Scholar]
- Fräki J. E., Hopsu-Havu V. K. Human skin proteases: separation and characterization of two alkaline proteases, one splitting trypsin and the other chymotrypsin substrates. Arch Dermatol Res. 1975 Oct 29;253(3):261–276. doi: 10.1007/BF00561152. [DOI] [PubMed] [Google Scholar]
- Fräki J. E. Human skin proteases. Separation and characterization of two acid proteases resembling cathepsin B1 and cathepsin D and of an inhibitor of cathepsin B1. Arch Dermatol Res. 1976 Jun 21;255(3):317–330. doi: 10.1007/BF00561502. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Wu C. D., Blumenthal H. J. Characterization of L-leucine-induced germination of Trichophyton mentagrophytes microconidia. J Bacteriol. 1972 Nov;112(2):967–976. doi: 10.1128/jb.112.2.967-976.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Dillavou C. L., Greenberg J. H., Jeppsen J. C., Jaegar J. S. Identification of carbon dioxide as a dermatophyte inhibitory factor produced by Candida albicans. Can J Microbiol. 1976 Dec;22(12):1720–1727. doi: 10.1139/m76-254. [DOI] [PubMed] [Google Scholar]
- Merz W. G., Lloyd K. O., Silva-Hutner M. Characterization of a Trichophyton mentagrophytes and its diffusible pigment. Sabouraudia. 1972 Mar;10(1):86–93. [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Neurospora crassa conidial germination: role of endogenous amino acid pools. J Bacteriol. 1975 Oct;124(1):232–242. doi: 10.1128/jb.124.1.232-242.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E. Freie Aminosäuren und verwandte Verbindungen in der abschabbaren Hornschicht unbefallener Haut von mikrobiellen Ekzematikern und Hautgesunden. Arch Dermatol Forsch. 1971;242(1):87–96. [PubMed] [Google Scholar]