Abstract
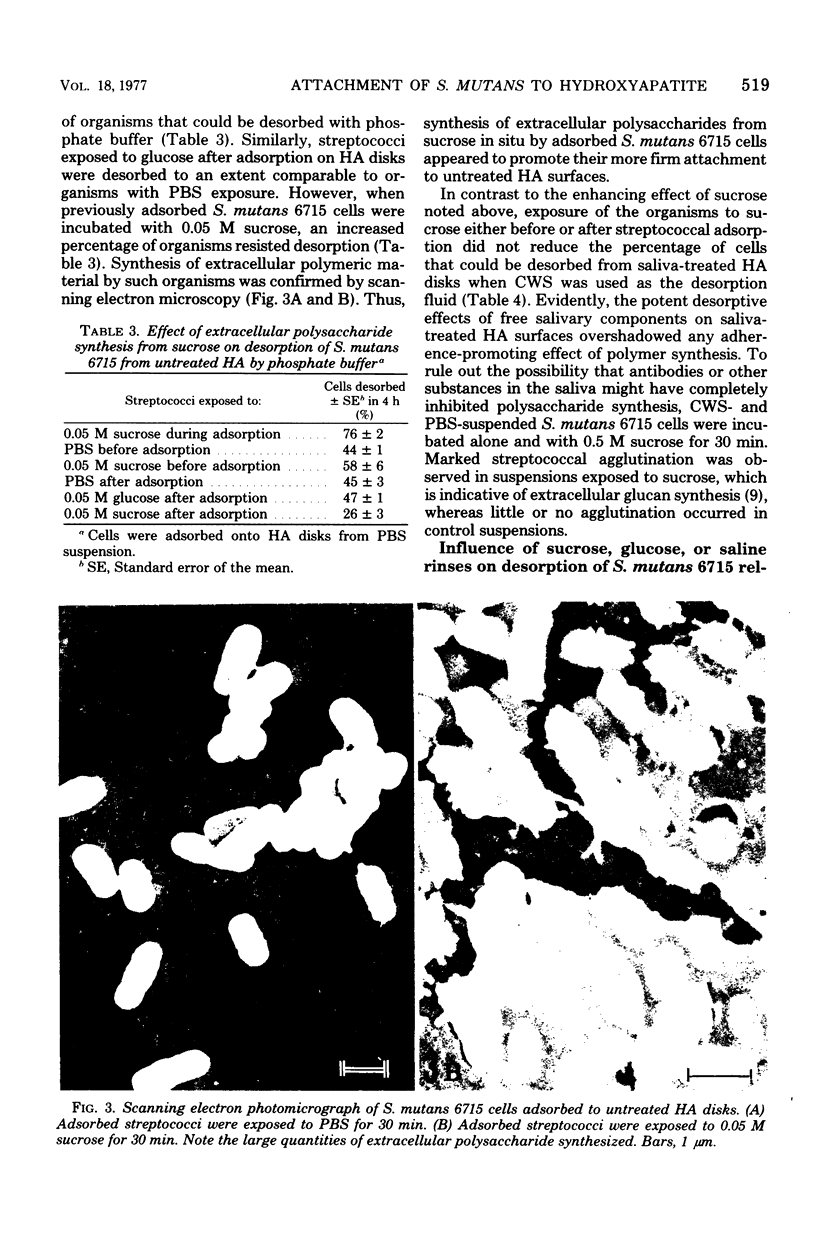
The adsorption of 3H-labeled Streptococcus mutans 6715 cells to disks of hydroxyapatite (HA) was studied. The number of streptococci that adsorbed was logarithmically related to the concentration of cells available up to at least 2 × 108 per ml; equilibrium occurred within 45 min. Assay reliability was verified by direct scanning electron microscopic counts. Untreated HA disks exposed to buffered saline (PBS)-suspended streptococci at a concentration of 1.1 × 108 per ml absorbed 3.2 × 106 cells per cm2; approximately 3% of the surface area was, therefore, occupied by adsorbed organisms. The presence of adsorbed salivary components on HA reduced the number of attaching S. mutans cells by half. When S. mutans cells were suspended in saliva to mimic conditions existing in the mouth, the number of streptococci adsorbing to saliva-treated HA was reduced more than 30-fold compared to untreated HA. Approximately one-half of the streptococci adsorbed to untreated or to saliva-treated HA disks could be desorbed over a 4-h period with 0.067 M phosphate buffer. S. mutans cells exposed to sucrose to permit extracellular polysaccharide synthesis before or during adsorption attached in fewer numbers to both saliva-treated and untreated HA than PBS-treated organisms. When S. mutans cells adsorbed on untreated HA were exposed to sucrose, fewer organisms could be desorbed; thus, in situ polysaccharide synthesis promoted their more firm attachment to untreated HA. However, when saliva-suspended streptococci were adsorbed to saliva-treated HA surfaces, exposure to sucrose before or subsequent to adsorption did not promote more firm attachment. Evidently, the powerful adherence-inhibiting and desorptive effects of salivary components overshadowed any promoting effects attributable to glucan synthesis from sucrose. Similarly, no differences were noted in the desorption of S. mutans cells from human teeth after exposure to sucrose, glucose, or PBS relative to a strain of Streptococcus mitis (S. mitior). Thus, no evidence was obtained to support the hypothesis that glucan synthesis from sucrose was essential for, or promoted, the attachment of S. mutans cells to HA surfaces exposed to saliva or to the smooth surfaces of human teeth.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Bratthall D., Gibbons R. J. Changing agglutination activities of salivary immunoglobulin A preparations against oral streptococci. Infect Immun. 1975 Mar;11(3):603–606. doi: 10.1128/iai.11.3.603-606.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Ericson T. Salivary glycoproteins. Composition and adsorption to hydroxylapatite in relation to the formation of dental pellicles and calculus. Acta Odontol Scand. 1968 May;26(1):3–21. doi: 10.3109/00016356809004577. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H. In vitro methods for the study of plaque formation and carious lesions. Arch Oral Biol. 1966 Aug;11(8):793–802. doi: 10.1016/0003-9969(66)90005-7. [DOI] [PubMed] [Google Scholar]
- KRASSE B. THE EFFECT OF THE DIET ON THE IMPLANTATION OF CARIES-INDUCING STREPTOCOCCI IN HAMSTERS. Arch Oral Biol. 1965 Mar-Apr;10:215–221. doi: 10.1016/0003-9969(65)90022-1. [DOI] [PubMed] [Google Scholar]
- Krasse B., Edwardsson S., Svensson I., Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967 Feb;12(2):231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- McGaughey C., Field B. D., Stowell E. C. Effects of salivary proteins on the adsorption of cariogenic streptococci by hydroxyapatite. J Dent Res. 1971 Jul-Aug;50(4):917–922. doi: 10.1177/00220345710500042201. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J., Krasse B. A method for studying adherence of oral streptococci to solid surfaces. Scand J Dent Res. 1976 Jan;84(1):20–28. doi: 10.1111/j.1600-0722.1976.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönju T., Christensen T. B., Kornstad L., Rölla G. Electron microscopy, carbohydrate analyses and biological activities of the proteins adsorbed in two hours to tooth surfaces in vivo. Caries Res. 1974;8(2):113–122. doi: 10.1159/000260099. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Upeslacis V. N., Jordan H. V., Skobe Z., Green D. B. Role of sucrose in colonization of Streptococcus mutans in conventional Sprague-Dawley rats. J Dent Res. 1976 Mar-Apr;55(2):202–215. doi: 10.1177/00220345760550020801. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Duchin S. Streptococcus mutans in the mouths of children with congenital sucrase deficiency. Arch Oral Biol. 1975 Nov;20(11):771–773. doi: 10.1016/0003-9969(75)90050-3. [DOI] [PubMed] [Google Scholar]



