Abstract
While the concept of a palpable relationship between our mental and physical well-being is certainly not new, it is only in the light of modern scientific research that we have begun to realize how deeply connected our emotional and immune states may be. We begin this review with a series of studies demonstrating how four fundamental emotional responses: anger, anxiety, mirth and relaxation are able modulate cytokine production and cellular responses to a variety of immune stimuli. These modulations are shown to be either detrimental or beneficial to a patient's health dependent on the context and duration of the emotion. We also discuss the reverse, highlighting research demonstrating how the loss of key immune cells such as T lymphocytes in clinical and animal studies can negatively impact both emotional well-being and cognition. Additionally, to give a more complete picture of the manifold pathways that link emotion and the immune system, we give a brief overview of the influence the digestive system has upon mental and immunological health. Finally, throughout this review we attempt to highlight the therapeutic potential of this burgeoning field of research in both the diagnosis and treatment of immune and disorders. As well as identifying some of the key obstacles the field must address in order to put this potential into practice.
Keywords: autoimmunity, emotion, immunosuppression, inflammation, mental health
Introduction: defunct medicine
“The mind most effectually works upon the body, producing by his passions and perturbations miraculous alterations … cruel diseases and sometimes death itself”. Robert Burton, The Anatomy of Melancholy, (1621/1893).
Humorism is an ancient medical theory and philosophy centred around the concept that differing combinations of four key bodily fluids (blood, phlegm, black and yellow bile) have distinctive effects on human health and behaviour. Although now widely discredited, for over 2000 years humorism dominated medical practice across much of the civilized world. One of this theory's most famous proponents was the ancient Greek philosopher and paragon of early medicine Hippocrates. His teachings on the subject were still practiced 700 years later during the height of the Roman empire, when the prominent surgeon (and also philosopher) Galen would use humorism as the basis for his hypothesis that a ‘balance of the passions’ was fundamental to good physical health. Indeed up until the late nineteenth century intense emotional states and responses (such as grief, anger and anxiety) were still considered a leading cause of human morbidity and mortality. The precepts of humorism pervaded western culture for such a long time that many of the words we use today to describe our own personalities, such as sanguine, phlegmatic and melancholy have their origins in this ancient medical doctrine.
While undoubtedly erroneous as a form of medicine (though bloodletting appears to be making something of a comeback1,2) a growing body of scientific evidence does back up a concept lying at the heart of humorist philosophy: that a link exists between emotional, physical and immunological health. In this review we will re-assess the Hippocratic theory of humorism in light of recent discoveries in the field of modern immunology, supporting the idea that changes in immune state can be detected by the nervous system and translated to a corresponding change in emotion. We will also examine the reverse: where distinct emotional states have been demonstrated to bring about alterations in the function of both the immune repertoire and response. Evidence will also be provided of pathophysiological conditions where an impaired emotional response could be considered a biomarker of physical disease. In doing so, we hope to highlight the potential therapeutic value of a patient's emotional state in the treatment of immune diseases, as well as speculate on the possibility of modulating the immune system to support mental well-being, most especially in the field of psychiatry.
Anger, laughter, stress and tai chi: emotion and the immune system
As well as its classical role in protecting against pathogens, the immune system has been established as playing a key part in governing homeostatic conditions linked to immune surveillance against inflammatory disease and tumorigenesis. Over several decades multiple regulatory pathways have been identified linking the immune with nervous and endocrine systems, mediated through the release of cytokines, endocrine hormones and neurotransmitter activity3,4 (Fig.1). In turn a number of endocrine factors (such as oxytocin, cortisol and noradrenaline) are known to be produced in response to various emotional stimuli.5–7 Many of these same stimuli have also been shown to elicit a striking effect on the immune response.
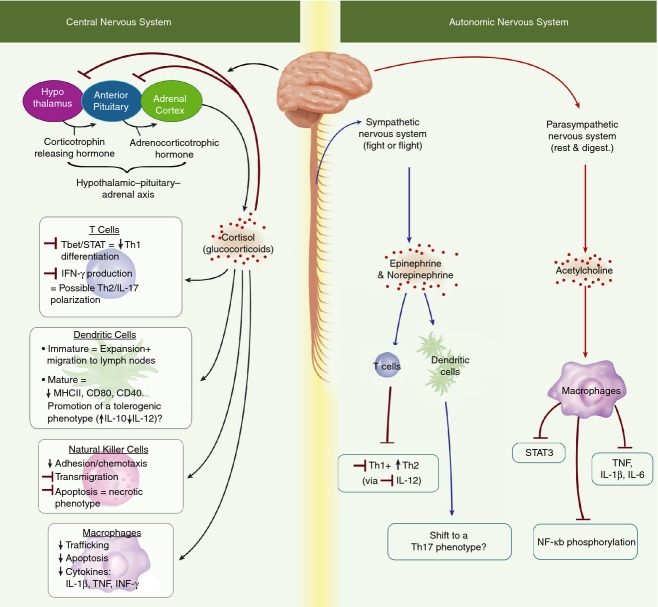
Figure 1.
The central nervous system (CNS) mediates the release of various immune influencing glucocorticoids via activation of a series of connected regions within the brain referred to as the hypothalamic–pituitary–adrenal axis. Research suggests the CNS to be primarily immune-suppressive in action, inhibiting production of pro-inflammatory cytokines, chemotactic factors and limiting migration and activation in several immune cell types.111–114 Additionally the CNS elicits differential immune effects dependent on cell type and stage of development, inducing the expansion and migration of immature dendritic cells while seemingly promoting a tolerogenic phenotype in their mature counterparts.113,115 Although less studied, several papers have demonstrated that the sympathetic nervous system is able to modulate immune activity through production of epinephrine and norepinephrine promoting a T helper type 2 (Th2) and Th17 phenotype in in T cells and dendritic cells, respectively.116,117 Produced by the parasympathetic nervous system, acetylcholine has been shown to interact directly with multiple immune cell subsets through expression of acetylcholine receptors, leading to suppression of a number of pro-inflammatory pathways in macrophages and other immune cells.118–120 Abbreviations: IFN-γ, interferon-γ; IL-17, interleukin-17; NF-κB, nuclear factor-κB; STAT, signal transducer and activator of transcription; Th1, T helper type 1; TNF, tumour necrosis factor.
Anger is a fundamental emotional state triggered reflexively in response to a perceived threat or provocation (this threat could be either physical or emotional). Multiple studies have explored the effects of anger on the immune system, together suggesting that this emotion can induce distinct yet related responses dependent on the context of the triggering event. For example anger associated with a hostile marital interaction has been shown to increase the production of the inflammatory cytokine interleukin-6 (IL-6) and circulating levels of C-reactive protein.8 In a different social setting, rugby players underwent psychological and serological evaluation 72 and 2 hr preceding an important match. A positive and independent relationship was identified between feelings of aggression, anger and anxiety (which for most increased between time-points) and circulating levels of IL-1β.9 Even the memory of anger-triggering events, elicited using an established technique referred to as the Anger Recall Interview,10 have been shown to significantly increase peripheral blood monocyte production of tumour necrosis factor-α (TNF-α) and IL-6 following lipopolysaccharide stimulation.11 These same cells were also shown to express significantly higher levels of α2-integrins in subjects that specifically had an increased anger response, as well as increased norepinephrine levels and accelerated diastolic blood pressure.12
One could speculate that in evolutionary terms the seemingly immunostimulatory (or immune-antagonistic) effect of anger is a beneficial one. For our ancestors anger often preceded physical violence, which in turn often resulted in injury. In these circumstances an immune system primed for action would certainly be advantageous, at least in the short term. However, further clinical and experimental studies have shown that in other instances, often when sensations of stress or anger become persistent, our immune response may become notably diminished or imbalanced. Individuals with below average levels of anger control were shown to heal significantly slower than subjects less disposed to this emotion.13 Similar observations were made in a study comparing differences in wound healing among students during their summer vacation and the run up to their exams. Recovery during the stressful exam period was on average 3 days slower than during vacation with a reported 68% reduction in IL-β production.14 In a number of studies Kiecolt-Glaser and colleagues have demonstrated that chronically stressful situations such as those experienced by caregivers can weaken the immune response, significantly diminishing antibody production against influenza and pneumococcal pneumonia vaccines and increasing the chance of latent herpes simplex virus flare ups.15–17 Further, in a range of clinical and experimental studies Dhabhar and his collaborators have also shown that chronic and acute stress can trigger both a deleterious and a protective T-cell response in a wide variety of immune-related disorders including cancer, allergy and post-operative recovery.18–25 As you will see throughout this review, there is little that is clear cut within this developing field.
Positive emotional states or emotional well-being (there is some debate in our laboratory as to whether any emotion could be categorized as singularly positive or negative) also have a distinct effect on the immune system. Research studying the effects of relaxing interventions on health has seen enormous growth in recent years (possibly because of the steep increase in recorded stress-related disorders). Seminal studies conducted by Gruzelier26,27 have shown that hypnosis and guided relaxation cause a significant modulation of the immune response, increasing the number of CD4-positive T cells while buffering the drop in natural killer (NK) and CD8 cells that occurred in human subjects experiencing stress or anxiety. T'ai chi ch'uan, an ancient Chinese martial art form that has recently come into vogue in western society has been subject to multiple studies investigating its psychological benefits and their effects on the immune system. When collated this research suggests that performing T'ai chi exercises leads to an improvement of both cell-mediated immunity and antibody response to infection.28–36 However, it should be noted that few studies have demonstrated a unified mechanism behind these improvements – instead focusing on specific players in the immune response such as circulating myeloid dendritic cells 28,37 or pro-inflammatory CD14+ CD16+ monocytes.38
Laughter has also been reported to have a surprisingly potent modulating effect on the immune system. Studies have shown that laughing therapy (in which at its most simple subjects are made to watch humorous films) up-regulates the expression of genes involved in the NK cell immune response, such as granzymes H and B, perforin, cathepsin and granulysin.39,40 Similar effects on NK cells have been described in further investigations where laughter was additionally shown to significantly decrease levels of circulating pro-inflammatory cytokines in patients with rheumatoid arthritis.41 It was further demonstrated that this therapy suppressed the heightened expression of growth hormone and insulin-like growth factor 1 that is often associated with the disease.42 In one of the first and more detailed studies on this subject, Berk et al. collected blood samples at regular time-points before and after a subject was exposed to a humorous video. Their results demonstrated that the potentiating effect of laughter on the immune system can last as long as 12 hr with increases in NK cell activity, immunoglobulin levels and functional phenotypic markers for multiple lymphocyte subsets.43 This persisting influence raises the question of the possible genomic effects of ‘happiness’, its molecular mechanisms of action and the possible therapeutic implications it may have. Of course this particular form of ‘mirthful laughter’ as Berk refers to it, is distinct from the laughter that can stem from emotions such as embarrassment and anxiety. It seems feasible that any immune response to this form of laughter may be distinct also.44
Leucocytes, lymphocytes, cognition and depression: the immune system and emotion
At the turn of the previous century the once presiding concepts of humorism underwent a dramatic decline. Primarily due to a spate of discoveries that began to reveal the architecture of both brain and body down to the cellular level. Most significantly the development of Magnetic resonance imaging by Nobel laureates Paul C Lauterbur and Peter Mansfield in 2003 allowed us to examine the flashes and pulses of brain activity in both real time and exquisite detail. Neuroscience has now progressed so far that we can now use it to predict the efficacy of psychological therapies. By assessing the occurrence of specific mutations in genes highly relevant to brain function such as nerve growth factor and brain-derived neurotrophic factor one group of researchers have successfully determined the efficacy of patient responses to cognitive behavioural therapy.45 This emerging field of research has been recently coined ‘therapygenetics’.46–48 Accompanying this upsurge of knowledge about the brain and its physical machinations has come an understandable preference to regard this organ as the sole instigator of human thought and feeling, processing events from our external environment into a set of defined emotional states, which in turn activate a corresponding physical response. While there is a wealth of scientific and epidemiological research supporting this top–down perspective of emotional reaction, there is a growing body of evidence to suggest the reverse: a bottom–up response in which events internal to our body yet separate from the brain may have a substantial influence on our mood.
In examining the influence immune cells may hold over the nervous system and brain let us first focus on the influential research brought forward by Kipnis and Schwartz.49–58 Their studies have demonstrated that depletion of CD4 T cells from adult mice positively correlates with impairment in learning and memory in the Morris's water maze test. Further investigation has established that populations of these cells accumulate in the meningeal space where they release IL-4. While classically a key immunoregulatory cytokine, IL-4 also seems to possess neuroprotective properties, up-regulating production of brain-derived neurotrophic factor, a neural hormone that promotes the growth of new neurons found in areas of the brain that are vital to learning, memory and higher thinking.54,59 This beneficial and homeostatic effect does not seem to be a unique requisite of CD4 T cells, extending to other cells producing the same cytokine such as M2 anti-inflammatory macrophages.60 However, a recent study has suggested that the beneficial effects of IL-4 may be reversed with ageing. Elderly mice displayed an excessive T helper type 2 response coupled with an increase in IL-4 production in their choroid plexus, a region of the brain responsible for the production of cerebrospinal fluid. The presence of this cytokine was shown to activate epithelial production of CCL-11, a chemokine known to be linked with cognitive dysfunction.61
Clinical studies, experimental evidence and patient accounts often describe the damaging effect of transitory or prolonged absence of T cells on emotional well-being. One of the best examples of T-cell-associated emotional imbalance in a disease condition is HIV/AIDS.62–65 In a study performed on 96 men without symptoms or previous treatment with retroviral medications, investigators found that stressful life events, dysphoric mood and limited social support were associated with more rapid clinical progression in HIV infection, with serum cortisol also exerting an independent effect on disease progression.66 Another comprehensive cross-sectional study expanded these observations, showing that the decreased numbers of T helper memory cell and B cells associated with high distress was mainly dependent on HIV viral load.67
Compounding the concept of T-cell-dependent mood modulation, striking alterations in human behaviour can also be induced by a wide range of immunomodulatory drugs. Cyclosporine, a T-cell-directed immunosuppressive peptide widely used in organ transplantation, has been shown to induce a range of neuropsychological problems including depression and anxiety both in patients and in experimental animals.68–70 Chemotherapy also commonly causes acute psychological complications, too severe to be considered a reaction to the treatments physical effects alone.71,72
While it seems clear that these cells have an influence on our emotional state, the key question that remains to be addressed is how and by what mechanisms? To this end we began investigating the impact of immunosuppression on emotional behaviour by performing classical behavioural tests on RAG1−/− mice.73 We additionally assessed the relative contribution of CD8 and CD4 cells to emotional impairment using RAG1−/−/OT-I and RAG1−/−/OT-II mice, respectively. Two previous studies, one by Cushman et al.74 and the other by McGowan et al.,75 have assessed the behavioural defects in these mice and reported a range of phenotypic differences including increased locomotor activity, reduced awareness of threat and impaired social recognition memory. Behaviours we considered analogous to a human state of acute anxiety. Our results complemented these observations and indicated an increased level of anxiety-like and obsessive compulsive behaviour, as demonstrated using the open field and marble burying tests.
A key issue facing thousands of individuals in western societies who currently suffer from anxiety and obsessive compulsive disorders is a diminished ability to perform basic day to day activities (e.g. cooking, cleaning, shopping).76–78 RAG1−/− mice present comparable problems, showing a significant decline in their ability to build their own nest and reduced social interaction with other animals. Our results with RAG1−/−/OT-I and RAG1−/−/OT-II mice also agreed with previous studies indicating that CD4 rather than CD8 T cells played the leading role in this cross-talk between the immune system and emotional behaviour.79–81 However, we did not observe comparable effects following a 3-week period of T-cell depletion in wild-type C57BL/6 mice. This led us to hypothesize that while impairment of cognition is a reversible and quickly ameliorated process that manifests following perturbation of the immune repertoire, a longer period of immunodeficiency might lead to a more lasting state level of behavioural dysfunction. Potentially under the influence of genetic control.
To this aim we sought to investigate if the immunodeficiency of RAG1−/− mice might have a persisting effect on the brain. Using micro-array analysis we were able to identify 111 genes that were specifically altered in these immune-deficient mice. Of these genes many have already been described as being directly involved in the progress or instigation of various neurodegenerative disorders including Huntington's, Alzheimer's and Parkinson's diseases (all of which cause a deficit in cognition and an impaired emotional state). Most strikingly these differences in gene expression were strongly reduced or abolished completely in the brains of RAG1−/−/OT-II mice, providing what we believe is exciting evidence that these cells may be arbiters of both immunity and emotion.
Gut feelings: immunity, emotion and the digestive system
As a final point let us briefly examine the human digestive system, which has long been known to have a somewhat enigmatic relationship with our emotional health.82–85 A connection between persistent stress and an increased risk of suffering gastrointestinal disorders has become so established that these maladies are often treated with anti-depressants or behavioural therapies.83,86–88 This brain–gut axis, as it is often described,89–91 has experienced a recent upsurge in research focus, with one group going so far as to examine the validity of that commonly given advice to ‘follow your gut’ (the conclusion being ‘yes’ albeit with several provisos).92,93 Multiple studies published last year have suggested the presence of particular ‘melancholic’ fauna in our bowel94 or the absence of particular probiotics95,96 has been linked to altered states of mental health and cognition. Additionally germ-free mice (which lack an intestinal micro-fauna) display a highly exaggerated response to stressful stimuli when compared with those with a normal compliment of gut bacteria.82,97 These findings are supported by a recent letter to the journal Nature, which has demonstrated that a functioning microbiota is essential for correct social development in mice.98 Mice lacking any gut bacteria from birth have also been reported to possess a substantially underdeveloped immune system.99,100 An unsurprising phenotype perhaps, if one analogizes the immune system to a muscle that must be trained to develop correctly. Corroborating this work, a study by a team of French scientists has demonstrated that the effects of the immunosuppressive anticancer drug cyclophosphamide are ablated in germ-free mice. The authors suggested that the absence of a gut microbiota in these animals prevented the induction of the specific subset of pathogenic T helper type 17 cells and memory T helper type 1 cells that would target the tumour in normal mice.101 An interesting follow-up study might investigate if the microbiota uses the immune system to influence mood and emotions, such as the way parasitic helminths have been shown to decrease aggression and short-term memory in infected mice.102 Finally, studies into the ‘hunger hormone’ ghrelin have revealed that this peptide not only plays a significant role in appetite regulation and energy homeostasis but also exerts a significant modulatory effect on both mood and the immune system. Ghrelin-treated mice exhibit reduced inflammation and enhanced lymphocyte development in response to immune stimuli, while ghrelin knockout animals presented heightened anxiety and depressive behaviour when subjected to a variety of emotional stressors.103–105 Research such as this offers a compelling hint that the nervous, digestive and immune systems are considerably more interconnected and overlapping than previously thought.
Conclusion: above, below and ahead
These studies and numerous others make a strong case for the immune system serving as both a channel and a controller of our emotional state. Regardless, this field remains a very new (or at least very recently renewed) area of research and there is much still to be established.
Foremost, we must consolidate the idea that the immune system can be influenced by the social (not just the physical) environment. A key foundation of this should be a rigorous investigation into how drug-free psychosocial treatments can influence the immune response. There is substantial evidence that the social context of patients suffering psychiatric disorders greatly influences the outcome of any pharmacological treatment they may receive. Seminal work by Priebe et al.106 has shown how aspects of treatment including how psychiatrists introduce themselves or how many friends the psychiatric patient has107 are significant factors that influence the outcome of therapy. Hence, if the future of psychiatry is social, as Priebe et al.108 have suggested, is it possible that the treatment of immune diseases might also include this consideration? Second, a great deal of systematic research (with standardized treatments and techniques) is needed to support these hypotheses and also to address the often conflicting results that this research has generated. Steps in this direction can already be found in the literature. As an example, in a comprehensive study by Marazziti et al.109 the long-term (12 months) treatment of adult obsessive compulsive disorder patients with selective serotonin reuptake inhibitors significantly reversed the skewed CD4/CD8 ratio (increase of CD8 and decrease of CD4) that was observed at baseline. This reversal occurred in parallel with the patient's clinical improvement.
Recent estimates by the World Health Organization suggest that by 2030 depression and stress-related problems will be the most debilitating and widespread health disorders on the planet, closely followed (rather tellingly) by autoimmune disease and allergy.110 With growing evidence that our emotional and immune states share a complex and bi-directional relationship with one another we believe the time is ripe to begin a serious cross-field examination of the significance of these interdependent states. It is our hope for the future that greater credence may be given to both the physical and emotional wellbeing of a patient when trying give a prognosis. And that the collaboration of clinicians, psychologists and immunologists to understand and ameliorate disease of any kind, be it mental or physical, becomes the norm. Because as that old adage tells us:
Happiness and healthiness go hand in hand.
Disclosures
The authors declare that they have no competing interests.
References
- 1.Equitani F, Equitani F, Fernandez-Real J, Menichella G, Koch M, Calvani M, Nobili V, Mingrone G, Manco M. Bloodletting ameliorates insulin sensitivity and secretion in parallel to reducing liver iron in carriers of HFE gene mutations. Diabetes Care. 2008;31:3–8. doi: 10.2337/dc07-0939. [DOI] [PubMed] [Google Scholar]
- 2.Houschyar K, Houschyar K, Ludtke R, Dobos G, Kalus U, Broecker-Preuss M, Rampp T, Brinkhaus B, Michalsen A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Med. 2012;10:54–62. doi: 10.1186/1741-7015-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 4.Ziemssen T, Kern S. Psychoneuroimmunology – cross-talk between the immune and nervous systems. J Neurol. 2007;254:II8–11. doi: 10.1007/s00415-007-2003-8. [DOI] [PubMed] [Google Scholar]
- 5.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 6.Braw Y, Malkesman O, Merlender A, Bercovich A, Dagan M, Maayan R, Weizman A, Weller A. Stress hormones and emotion-regulation in two genetic animal models of depression. Psychoneuroendocrinology. 2006;31:1105–16. doi: 10.1016/j.psyneuen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Berk LS, Tan SA, Fry WF, Napier BJ, Lee JW, Hubbard RW, Lewis JE, Eby WC. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390–6. doi: 10.1097/00000441-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35:33–8. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesce M, Speranza L, Franceschelli S, et al. Positive correlation between serum interleukin-1β and state anger in rugby athletes. Aggress Behav. 2013;39:141–8. doi: 10.1002/ab.21457. [DOI] [PubMed] [Google Scholar]
- 10.Suarez EC, Saab PG, Llabre MM, Kuhn CM, Zimmerman E. Ethnicity, gender, and age effects on adrenoceptors and physiological responses to emotional stress. Psychophysiology. 2004;41:450–60. doi: 10.1111/j.1469-8986.00161.x. [DOI] [PubMed] [Google Scholar]
- 11.Suarez EC, Boyle SH, Lewis JG, Hall RP, Young KH. Increases in stimulated secretion of proinflammatory cytokines by blood monocytes following arousal of negative affect: the role of insulin resistance as moderator. Brain Behav Immun. 2006;20:331–8. doi: 10.1016/j.bbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Greeson JM, Lewis JG, Achanzar K, Zimmerman E, Young KH, Suarez EC. Stress-induced changes in the expression of monocytic β2-integrins: the impact of arousal of negative affect and adrenergic responses to the Anger Recall Interview. Brain Behav Immun. 2009;23:251–6. doi: 10.1016/j.bbi.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouin JP, Kiecolt-Glaser JK, Malarkey WB, Glaser R. The influence of anger expression on wound healing. Brain Behav Immun. 2008;22:699–708. doi: 10.1016/j.bbi.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–5. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19:78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- 16.Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000;62:804–7. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhabhar FS. Psychological stress and immunoprotection versus immunopathology in the skin. Clin Dermatol. 2013;31:18–30. doi: 10.1016/j.clindermatol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells–from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology. 2012;37:1345–68. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt D, Oberyszyn TM. High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One. 2012;7:e33069. doi: 10.1371/journal.pone.0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, Dhabhar FS. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009;91:2783–94. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin Immunol. 2008;4:2–11. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–13. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhabhar FS, Viswanathan K. Short-term stress experienced at time of immunization induces a long-lasting increase in immunologic memory. Am J Physiol Regul Integr Comp Physiol. 2005;289:R738–44. doi: 10.1152/ajpregu.00145.2005. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci USA. 2005;102:5808–13. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruzelier JH. The role of psychological intervention in modulating aspects of immune function in relation to health and well-being. Int Rev Neurobiol. 2002;52:383–417. doi: 10.1016/s0074-7742(02)52017-1. [DOI] [PubMed] [Google Scholar]
- 27.Gruzelier JH. A review of the impact of hypnosis, relaxation, guided imagery and individual differences on aspects of immunity and health. Stress. 2002;5:147–63. doi: 10.1080/10253890290027877. [DOI] [PubMed] [Google Scholar]
- 28.Ho RT, Wang CW, Ng SM, Ho AH, Ziea ET, Wong VT, Chan CL. The effect of t'ai chi exercise on immunity and infections: a systematic review of controlled trials. J Altern Complement Med. 2013;19:389–96. doi: 10.1089/acm.2011.0593. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Liu J, Chen P, Yu D. Regular tai chi exercise decreases the percentage of type 2 cytokine-producing cells in postsurgical non-small cell lung cancer survivors. Cancer Nurs. 2013;36:E27–34. doi: 10.1097/NCC.0b013e318268f7d5. [DOI] [PubMed] [Google Scholar]
- 30.Goon JA, Noor Aini AH, Musalmah M, Yasmin Anum MY, Wan Ngah WZ. Long term Tai Chi exercise reduced DNA damage and increased lymphocyte apoptosis and proliferation in older adults. Med J Malaysia. 2008;63:319–24. [PubMed] [Google Scholar]
- 31.Chen YY, Chiang J, Chen YJ, Chen KT, Yang RS, Lin JG. Cycling and Tai Chi Chuan exercises exert greater immunomodulatory effect on surface antigen expression of human hepatitis B virus. Chin Med J. 2008;121:2172–9. [PubMed] [Google Scholar]
- 32.Yeh SH, Chuang H, Lin LW, Hsiao CY, Wang PW, Liu RT, Yang KD. Regular Tai Chi Chuan exercise improves T cell helper function of patients with type 2 diabetes mellitus with an increase in T-bet transcription factor and IL-12 production. Br J Sports Med. 2009;43:845–50. doi: 10.1136/bjsm.2007.043562. [DOI] [PubMed] [Google Scholar]
- 33.Tai chi gives immune system a boost. Harv Health Lett. 2007;32:7. [PubMed] [Google Scholar]
- 34.Kobayashi H, Ishii M. Mind-body, Ki (Qi) and the skin: commentary on Irwin's ‘Shingles immunity and health functioning in the elderly: Tai Chi Chih as a behavioral treatment’. Evid Based Complement Alternat Med. 2005;2:113–6. doi: 10.1093/ecam/neh071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irwin M, Pike J, Oxman M. Shingles immunity and health functioning in the elderly: Tai Chi Chih as a behavioral treatment. Evid Based Complement Alternat Med. 2004;1:223–32. doi: 10.1093/ecam/neh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin MR, Pike JL, Cole JC, Oxman MN. Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med. 2003;65:824–30. doi: 10.1097/01.psy.0000088591.86103.8f. [DOI] [PubMed] [Google Scholar]
- 37.Chiang J, Chen YY, Akiko T, Huang YC, Hsu ML, Jang TR, Chen YJ. Tai Chi Chuan increases circulating myeloid dendritic cells. Immunol Invest. 2010;39:863–73. doi: 10.3109/08820139.2010.503766. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Geib RW. Exploring the use of five color flow cytometry to examine the effect of acute tai chi practice on pro inflammatory monocyte subtypes – biomed 2013. Biomed Sci Instrum. 2013;49:209–15. [PubMed] [Google Scholar]
- 39.Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185–7. doi: 10.1016/j.lfs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi T, et al. Laughter up-regulates the genes related to NK cell activity in diabetes. Biomed Res. 2007;28:281–5. doi: 10.2220/biomedres.28.281. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki T, Nakajima A, Ishigami S, Tanno M, Yoshino S. Mirthful laughter differentially affects serum pro- and anti-inflammatory cytokine levels depending on the level of disease activity in patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45:182–6. doi: 10.1093/rheumatology/kei081. [DOI] [PubMed] [Google Scholar]
- 42.Ishigami S, Nakajima A, Tanno M, Matsuzaki T, Suzuki H, Yoshino S. Effects of mirthful laughter on growth hormone, IGF-1 and substance P in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005;23:651–7. [PubMed] [Google Scholar]
- 43.Berk LS, Felten DL, Tan SA, Bittman BB, Westengard J. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001;7:62–72. 74–6. [PubMed] [Google Scholar]
- 44.Berk LS. Studying the biology of hope: an interview with Lee S. Berk, DrPH, MPH. Interview by Sheldon Lewis. Adv Mind Body Med. 2007;22:28–31. [PubMed] [Google Scholar]
- 45.Lester KJ, Hudson JL, Tropeano M, et al. Neurotrophic gene polymorphisms and response to psychological therapy. Transl Psychiatry. 2012;2:e108. doi: 10.1038/tp.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lester KJ, Eley TC. Therapygenetics: using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol Mood Anxiety Disord. 2013;3:4. doi: 10.1186/2045-5380-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bockting CL, Mocking RJ, Lok A, Koeter MW, Schene AH. Therapygenetics: the 5HTTLPR as a biomarker for response to psychological therapy? Mol Psychiatry. 2013;18:744–5. doi: 10.1038/mp.2012.92. [DOI] [PubMed] [Google Scholar]
- 48.Eley TC, Hudson JL, Creswell C, et al. Therapygenetics: the 5HTTLPR and response to psychological therapy. Mol Psychiatry. 2012;17:236–7. doi: 10.1038/mp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014;141:340–4. doi: 10.1111/imm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–96. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin I, Kipnis J. Learning and memory… and the immune system. Learn Mem. 2013;20:601–6. doi: 10.1101/lm.028357.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derecki NC, Kipnis J. From neurons to microglia, with complements. Nat Neurosci. 2013;16:1712–3. doi: 10.1038/nn.3579. [DOI] [PubMed] [Google Scholar]
- 53.Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–9. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–9. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6:405–10. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–42. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Villoslada P, Moreno B, Melero I, et al. Immunotherapy for neurological diseases. Clin Immunol. 2008;128:294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2011;25:379–85. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baruch K, Ron-Harel N, Gal H, et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci USA. 2013;110:2264–9. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole SW, Kemeny ME, Taylor SE, Visscher BR, Fahey JL. Accelerated course of human immunodeficiency virus infection in gay men who conceal their homosexual identity. Psychosom Med. 1996;58:219–31. doi: 10.1097/00006842-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Kemeny ME, Dean L. Effects of AIDS-related bereavement on HIV progression among New York City gay men. AIDS Educ Prev. 1995;7:36–47. [PubMed] [Google Scholar]
- 64.Rabkin JG, Wagner G, Rabkin R. Effects of sertraline on mood and immune status in patients with major depression and HIV illness: an open trial. J Clin Psychiatry. 1994;55:433–9. [PubMed] [Google Scholar]
- 65.Rabkin JG, Rabkin R, Wagner G. Effects of fluoxetine on mood and immune status in depressed patients with HIV illness. J Clin Psychiatry. 1994;55:92–7. [PubMed] [Google Scholar]
- 66.Leserman J, Petitto JM, Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32:1059–73. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 67.Motivala SJ, Hurwitz BE, Llabre MM, Klimas NG, Fletcher MA, Antoni MH, LeBlanc WG, Schneiderman N. Psychological distress is associated with decreased memory helper T-cell and B-cell counts in pre-AIDS HIV seropositive men and women but only in those with low viral load. Psychosom Med. 2003;65:627–35. doi: 10.1097/01.psy.0000041549.72780.5b. [DOI] [PubMed] [Google Scholar]
- 68.Pudlo R, Piegza M, Zakliczynski M, Zembala M. The occurrence of mood and anxiety disorders in heart transplant recipients. Transpl Proc. 2009;41:3214–8. doi: 10.1016/j.transproceed.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 69.Bahi A, Mineur YS, Picciotto MR. Blockade of protein phosphatase 2B activity in the amygdala increases anxiety- and depression-like behaviors in mice. Biol Psychiatry. 2009;66:1139–46. doi: 10.1016/j.biopsych.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Akaho R, Sasaki T, Mori S, Akiyama H, Yoshino M, Hagiya K, Nakagome K, Sakamaki H. Psychological factors and survival after bone marrow transplantation in patients with leukemia. Psychiatry Clin Neurosci. 2003;57:91–6. doi: 10.1046/j.1440-1819.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 71.Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140. doi: 10.1186/1471-244X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D'Acquisto F. CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10:13–8. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Res. 2011;1383:187–95. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bystritsky A, Liberman RP, Hwang S, Wallace CJ, Vapnik T, Maindment K, Saxena S. Social functioning and quality of life comparisons between obsessive-compulsive and schizophrenic disorders. Depress Anxiety. 2001;14:214–8. doi: 10.1002/da.1069. [DOI] [PubMed] [Google Scholar]
- 77.Huppert JD, Simpson HB, Nissenson KJ, Liebowitz MR, Foa EB. Quality of life and functional impairment in obsessive-compulsive disorder: a comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26:39–45. doi: 10.1002/da.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lochner C, Mogotsi M, du Toit PL, Kaminer D, Niehaus DJ, Stein DJ. Quality of life in anxiety disorders: a comparison of obsessive-compulsive disorder, social anxiety disorder, and panic disorder. Psychopathology. 2003;36:255–62. doi: 10.1159/000073451. [DOI] [PubMed] [Google Scholar]
- 79.Cohen H, et al. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+ CD25+ cells. J Neurobiol. 2006;66:552–63. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- 80.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–5. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–84. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 82.Nishino R, Mikami K, Takahashi H, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25:521–8. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 83.Bonaz BL, Bernstein CN. Brain–gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Cizza G, Rother KI. Was Feuerbach right: are we what we eat? J Clin Investig. 2011;121:2969–71. doi: 10.1172/JCI58595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Oudenhove L. Visceral sensory and cognitive-affective neuroscience: towards integration? Gut. 2010;59:431–2. doi: 10.1136/gut.2009.192658. [DOI] [PubMed] [Google Scholar]
- 86.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–14. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fennerty MB. Traditional therapies for irritable bowel syndrome: an evidence-based appraisal. Rev Gastroenterol Disord. 2003;3(Suppl. 2):S18–24. [PubMed] [Google Scholar]
- 88.Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313–27. doi: 10.1016/s0025-7125(05)70288-1. [DOI] [PubMed] [Google Scholar]
- 89.Fichna J, Storr MA. Brain–gut interactions in IBS. Front Pharmacol. 2012;3:127. doi: 10.3389/fphar.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bercik P, Collins SM, Verdu EF. Microbes and the gut–brain axis. Neurogastroenterol Motil. 2012;24:405–13. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 91.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain–gut–microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stolper E, Van de Wiel M, Van Royen P, Van Bokhoven M, Van der Weijden T, Dinant GJ. Gut feelings as a third track in general practitioners' diagnostic reasoning. J Gen Intern Med. 2011;26:197–203. doi: 10.1007/s11606-010-1524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mikels JA, Maglio SJ, Reed AE, Kaplowitz LJ. Should I go with my gut? Investigating the benefits of emotion-focused decision making. Emotion. 2011;11:743–53. doi: 10.1037/a0023986. [DOI] [PubMed] [Google Scholar]
- 94.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713–9. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 95.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–61. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 96.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desbonnet L, Clarke G, Shanahan F, Dinan T, Cryan J. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2013;19:146–8. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hansen CH, Metzdorff SB, Hansen AK. Customizing laboratory mice by modifying gut microbiota and host immunity in an early “window of opportunity”. Gut Microbes. 2013;4:241–5. doi: 10.4161/gmic.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–20. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morales-Montor J, Picazo O, Besedovsky H, et al. Helminth infection alters mood and short-term memory as well as levels of neurotransmitters and cytokines in the mouse hippocampus. NeuroImmunoModulation. 2014;21:195–205. doi: 10.1159/000356521. [DOI] [PubMed] [Google Scholar]
- 103.Aylwin S, Al-Zaman Y. Emerging concepts in the medical and surgical treatment of obesity. Front Horm Res. 2008;36:229–59. doi: 10.1159/000115368. [DOI] [PubMed] [Google Scholar]
- 104.Lago F, Gonzalez-Juanatey JR, Casanueva FF, Gómez-Reino J, Dieguez C, Gualillo O. Ghrelin, the same peptide for different functions: player or bystander? Vitam Horm. 2005;71:405–32. doi: 10.1016/S0083-6729(05)71014-1. [DOI] [PubMed] [Google Scholar]
- 105.Spencer SJ. Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB. Ghrelin regulates the hypothalamic–pituitary–adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72:457–65. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Priebe S, Palumbo C, Ahmed S, Strappelli N, Gavrilovic JJ, Bremner S. How psychiatrists should introduce themselves in the first consultation: an experimental study. Br J Psychiatry. 2013;202:459–62. doi: 10.1192/bjp.bp.112.123877. [DOI] [PubMed] [Google Scholar]
- 107.Giacco D, McCabe R, Kallert T, Hansson L, Fiorillo A, Priebe S. Friends and symptom dimensions in patients with psychosis: a pooled analysis. PLoS One. 2012;7:e50119. doi: 10.1371/journal.pone.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Priebe S, Burns T, Craig TK. The future of academic psychiatry may be social. Br J Psychiatry. 2013;202:319–20. doi: 10.1192/bjp.bp.112.116905. [DOI] [PubMed] [Google Scholar]
- 109.Marazziti D, Mungai F, Masala I, et al. Normalisation of immune cell imbalance after pharmacological treatments of patients suffering from obsessive-compulsive disorder. J Psychopharmacol. 2009;23:567–73. doi: 10.1177/0269881108089605. [DOI] [PubMed] [Google Scholar]
- 110.Mathers C, Fat DM, Boerma J. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 111.Wingett D, Forcier K, Nielson CP. Glucocorticoid-mediated inhibition of RANTES expression in human T lymphocytes. FEBS Lett. 1996;398:308–11. doi: 10.1016/s0014-5793(96)01238-0. [DOI] [PubMed] [Google Scholar]
- 112.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 113.Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 114.van Oosten MJ, Dolhain RJ, Koper JW, et al. Polymorphisms in the glucocorticoid receptor gene that modulate glucocorticoid sensitivity are associated with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R159. doi: 10.1186/ar3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roca L, Di Paolo S, Petruzzelli V, Grandaliano G, Ranieri E, Schena FP, Gesualdo L. Dexamethasone modulates interleukin-12 production by inducing monocyte chemoattractant protein-1 in human dendritic cells. Immunol Cell Biol. 2007;85:610–6. doi: 10.1038/sj.icb.7100108. [DOI] [PubMed] [Google Scholar]
- 116.Kim B-J, Jones HP. Epinephrine-primed murine bone marrow-derived dendritic cells facilitate production of IL-17A and IL-4 but not IFN-γ by CD4+ T cells. Brain Behav Immun. 2010;24:1126–36. doi: 10.1016/j.bbi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panina-Bordignon P, Mazzeo D, Lucia P, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. β2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 119.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin Exp Immunol. 2006;146:116–23. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–7. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]