Abstract
Drug addiction is a chronically relapsing disorder characterized by loss of control over intake and dysregulation of stress-related brain emotional systems. Since the discovery by Wylie Vale and his colleagues of corticotropin-releasing factor (CRF) and the structurally-related urocortins, CRF systems have emerged as mediators of the body’s response to stress. Relatedly, CRF systems have a prominent role in driving addiction via actions in the central extended amygdala, producing anxiety-like behavior, reward deficits, excessive, compulsive-like drug self-administration and stress-induced reinstatement of drug seeking. CRF neuron activation in the medial prefrontal cortex may also contribute to the loss of control. Polymorphisms in CRF system molecules are associated with drug use phenotypes in humans, often in interaction with stress history. Drug discovery efforts have yielded brain-penetrant CRF1 antagonists with activity in preclinical models of addiction.. The results support the hypothesis that brain CRF-CRF1 systems contribute to the etiology and maintenance of addiction.
Keywords: corticotropin-releasing factor or hormone receptor antagonist, CRF or CRH or urocortin 1 or urocortin 2 or urocortin 3, anxiety disorder, major depression, alcohol or ethanol, drug addiction or alcoholism or alcohol dependence or alcohol use disorder or binge drinking, acute or protracted withdrawal or abstinence, treatment or clinical trial, stress-induced relapse or reinstatement or craving
Introduction
According to a 2012 report by the Substance Abuse and Mental Health Services Administration, within the past 12 months, approximately 15% of the population aged 12 and older experienced substance use disorders on alcohol, cigarettes, or an illegal drug. Alcohol use disorders alone have an annual prevalence of approximately 10% and account for 4.6% of all disability-adjusted life-years in developed countries (Rehm et al., 2009). Available pharmacotherapies for substance use disorders have only modest long-term efficacy and are underutilized (Heilig et al., 2011). Since the successive discovery by Wylie Vale and his colleagues of corticotropin-releasing factor (CRF) (Vale et al., 1981), the structurally-related urocortins (Ucn 1, Ucn 2, Ucn 3), and their cognate receptors (CRF1, CRF2) (Bale and Vale, 2004;Fekete and Zorrilla, 2007), CRF systems have emerged as therapeutic targets for substance abuse.
CRF binds with high and moderate potency to CRF1 and CRF2 receptors, respectively. Ucn 1 is a high-affinity agonist at both of these G-protein coupled receptors, whereas the type 2 urocortins (Ucn 2 and Ucn 3) are selective CRF2 receptor agonists (Bale and Vale, 2004;Zorrilla and Koob, 2004;Fekete and Zorrilla, 2007). Vale and colleagues first demonstrated that CRF initiates the hypothalamic-pituitary-adrenal (HPA) axis neuroendocrine stress response by binding CRF1 receptors in the anterior pituitary after release into portal blood. In addition, however, CRF1 receptors are widely distributed in stress-responsive brain regions, including the neocortex, central extended amygdala, medial septum, hippocampus, thalamus, cerebellum, and autonomic midbrain and hindbrain nuclei (Grigoriadis et al., 1996; Primus et al., 1997;Sanchez et al., 1999;Van Pett et al., 2000). The brain CRF1 receptor distribution resembles the distribution of its natural ligands CRF (Fig. 1) and Ucn 1 and accounts for the dissociable, non-endocrine role of extrahypothalamic CRF1 systems (i.e., outside the HPA axis) to mediate behavioral and autonomic stress responses (Swanson et al., 1983;Kozicz et al., 1998;Bale and Vale, 2004;Zorrilla and Koob, 2004;Fekete and Zorrilla, 2007).
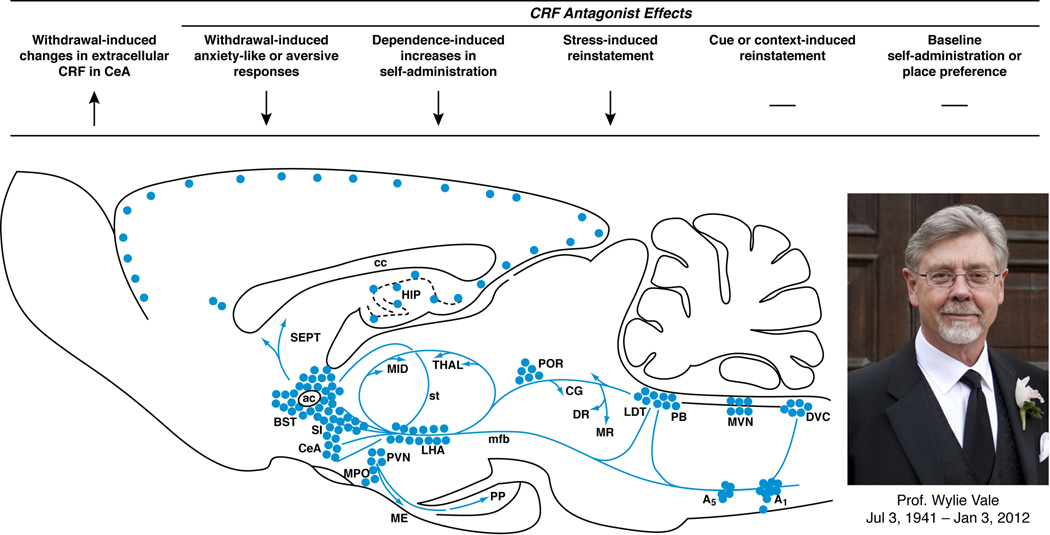
Figure 1. Brain CRF mediates the facilitation of compulsive-like drug use.
As shown in the sagittal brain schematic, corticotropin-releasing factor (CRF), first isolated by Professor Wylie Vale (photo), is expressed in neuronal cell bodies (filled circles) and projections (blue arrows) that subserve behavioral, autonomic and neuroendocrine responses to stress. As summarized from left-to-right by the arrows, CRF systems play an integral role in regulating the intersection between drug self-administration and stress systems. For example, drug or alcohol withdrawal elevates CRF activity in the central extended amygdala, including the central nucleus of the amygdala (CeA), leading to a negative emotional state that motivates resumption of and maintenance of drug-taking. Pharmacological studies with CRF antagonists show that increased CRF-CRF1 system activation underlies several withdrawal-induced behavioral phenotypes, including anxiety-like behavior, aversion, and elevated drug self-administration. CRF1 antagonists also reduce the effect of acute stressors on drug-related behaviors, including stress-induced reinstatement. In contrast, CRF1 antagonists do not alter non-stress mechanisms that reinstate drug-seeking, such as drug primes, cues or contexts, reflecting the distinct neuroanatomical substrates of relapse behavior. Blockade of CRF-CRF1 systems does not have intrinsic rewarding (or aversive) properties in place conditioning models and has little effect on baseline intake in nondependent individuals.
Extensive preclinical data suggest that extrahypothalamic CRF1 systems subserve negative emotional states. Accordingly, small-molecule CRF1 antagonists are being developed as potential treatments for affective-like disorders, including posttraumatic stress disorder, irritable bowel syndrome, anxiety disorders, and major depression (Zorrilla and Koob, 2004;Holsboer and Ising, 2008;Zorrilla and Koob, 2010;Koob and Zorrilla, 2012;Zorrilla et al., 2013a). Indeed, Dr. Vale was a major force in the pharmaceutical development of drug-like small-molecule CRF1 antagonists. He co-founded Neurocrine Biosciences, which successfully developed a wide range of such compounds, spanning multiple patents.
One proposed clinical indication for CRF1 antagonists is drug addiction (Fig. 1), where the brain stress systems are hypothesized to impact key elements of the addiction cycle. Drug addiction is a chronically relapsing disorder characterized by loss of control over drug intake and emergence of a negative emotional state during abstinence. Drug addiction has been conceptualized as a cycle progressing through three stages—binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation—that become worse over time and ultimately lead to a severe neurobiological disorder. CRF systems are hypothesized to play a key role in all three stages of the addiction cycle but particularly in the withdrawal/negative affect stage. Chronic use of a drug of abuse, even if initiated for its rewarding effects, increasingly leads to negative emotional symptoms and negatively reinforced substance use. An extension of the “opponent process theory of affective regulation” (Solomon and Corbit, 1974), this hypothesis of addiction proposes that drugs of abuse initially activate brain structures that subserve positive emotional states (e.g., pleasure, contentment). The positive reinforcing effects of drugs are regulated in part by the ventral striatum and extended amygdala reward system, as well as by dopaminergic and opioid inputs from the ventral tegmental area (VTA) and arcuate nucleus of the hypothalamus, respectively. To maintain emotional homeostasis, however, a counter-regulatory opponent process then decreases mood and increases vigilance/tension via downregulation of brain reward systems (e.g., ventral striatum) and upregulation of brain stress systems, including CRF and norepinephrine systems in the extended amygdala (Heilig and Koob, 2007;Heilig et al., 2010a;Heilig et al., 2010b;Koob and Zorrilla, 2010;Breese et al., 2011;Heilig et al., 2011;Logrip et al., 2011;Koob and Zorrilla, 2012). With continued cycles of intoxication/withdrawal, the opponent process allostatically predominates over the primary rewarding process (Fig. 2). As a result, more substance of abuse is needed simply to maintain euthymia. If drug use stops, negative emotional symptoms emerge (i.e., acute withdrawal: anxiety, dysphoria, irritability). With a sufficient drug use history, stress-like symptoms of dysphoria may episodically and spontaneously resurge even weeks or months after detoxification (i.e., protracted withdrawal). Furthermore, exaggerated responses to otherwise mild stressors may be seen despite continued abstinence. Under this conceptualization of addiction, substance abuse escalates because the drug of abuse mitigates the counter-regulatory negative emotional symptoms of acute and protracted withdrawal (Heilig and Koob, 2007;Koob and Zorrilla, 2010;Zorrilla et al., 2013a).
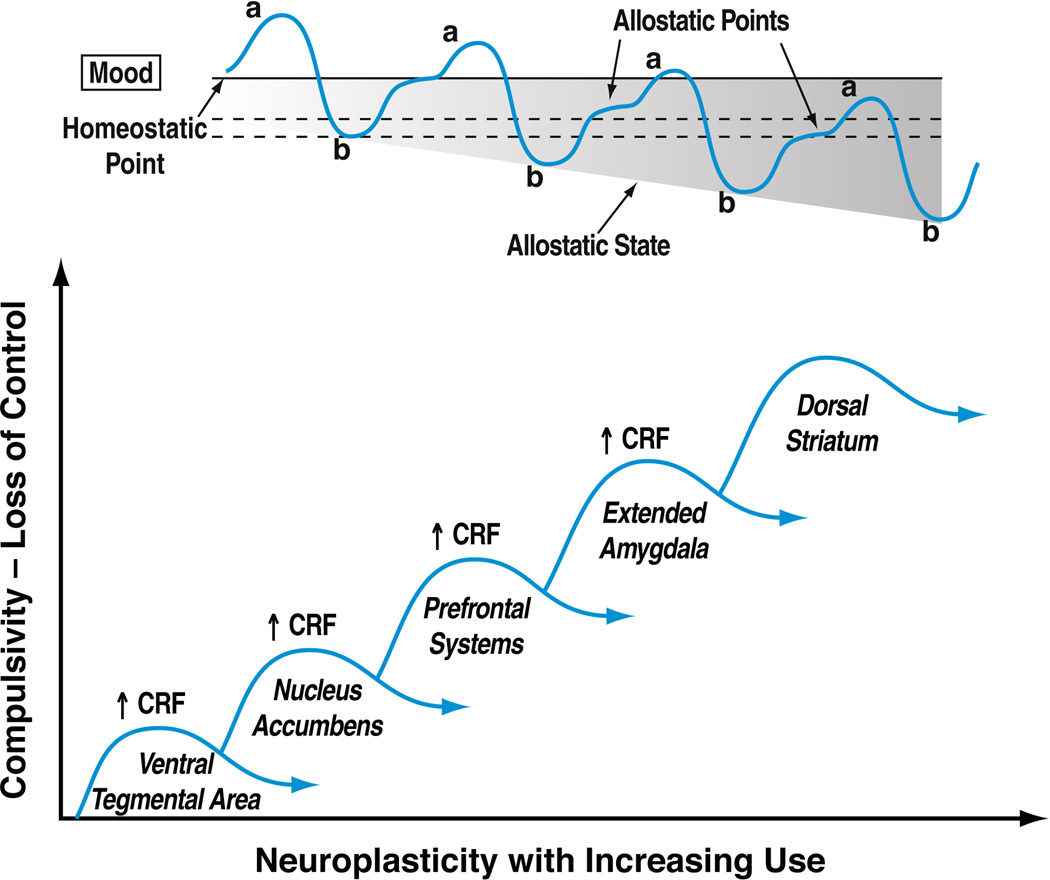
Figure 2. The progression to compulsive drug use alters emotional homeostasis via opponent-process upregulation of CRF activity in multiple brain nuclei.
The top-panel illustrates the opponent-process, emotional allostasis model of drug addiction. In the model, acute drug use initially elicits positive shifts in mood (“a” process). This is subsequently countered, however, by homeostatic decrements in mood (“b” opponent-process). With repeated drug use, the “b” opponent-process manifests earlier and more prominently, such that each drug experience elicits a smaller, briefer positive shift in mood. The associated neuroadaptations alter the individual’s emotional set points, yielding allostatic states of decreased reward function and increased stress function. The individual’s mood in a drug-free state does not return to the drug-naïve baseline (homeostatic point). Rather, new, stable allostatic states result, in which the drug-free baseline mood (allostatic points) becomes increasingly more negative, experienced as dysphoria in the absence of drug. With continued use, further drug-taking can no longer reattain the baseline, drug-naïve homeostatic set point, let alone a subjective positive “high” (Koob and Le Moal, 2001). Compulsive, escalating drug use is motivated by the negative emotional allostatic state in negative reinforcement fashion in a futile attempt to reduce dysphoria and regain euthymia. The bottom panel hypothesizes the successive recruitment of CRF systems across different brain regions during the transition to compulsive drug-taking and loss of control. During the initial phases of drug self-administration, when drug-taking still effectively elicits positive mood states and maintained by positive reinforcement, brain regions central to reward processes (ventral tegmental area, nucleus accumbens) are recruited. With continued drug use, neuroadaptations in CRF systems in the ventral tegmental area and prefrontal cortex may contribute to the generation of compulsive drug seeking. Finally, opponent-process activation of CRF systems in the extended amygdala and recruitment of circuitry linked to the dorsal striatum underlie the negative emotional state and habitual drug-seeking, respectively, observed in individuals with severe addiction.
The reviewed opponent process putatively of otherwise silent brain CRF1 receptor stress systems of the extended amygdala. For example, in dependent rat models, acute alcohol withdrawal activates CRF systems in the central nucleus of the amygdala (CeA) (Merlo Pich et al., 1995;Zorrilla et al., 2001;Funk et al., 2006;Roberto et al., 2010) and bed nucleus of the stria terminalis (Olive et al., 2002). Extracellular CRF in rats also increased in the CeA during precipitated withdrawal from chronic nicotine (George et al., 2007), withdrawal from binge cocaine self-administration (Richter and Weiss, 1999), and precipitated withdrawal from opioids (Weiss et al., 2001) and cannabinoids (Rodriguez de Fonseca et al., 1997). Nicotine withdrawal in rats also increased CeA CRF mRNA and, especially in females, NAc CRF mRNA levels (Aydin et al., 2011;Torres et al., 2013). Conversely, amygdala CRF tissue content is reduced during acute withdrawal from ethanol exposure (Zorrilla et al., 2001;Funk et al., 2006;Wills et al., 2010) and from binge cocaine self-administration (Zorrilla et al., 2001;Zorrilla et al., 2012), suggesting degradation and depletion after sustained secretion. Supporting a functional role for central extended amygdala CRF1 receptor activation in the negative affect/withdrawal stage, site-specific injections of CRF receptor antagonists into the central amygdala reduce anxiety-like behavior, motivational deficits for other reinforcers, and excessive self-administration of addictive substances during acute withdrawal (Heilig and Koob, 2007;Heilig et al., 2010b;Koob and Zorrilla, 2010;Logrip et al., 2011;Parylak et al., 2011).
The opponent process also may involve pituitary CRF1-dependent activation of the HPA-axis, reflected by elevated ACTH and corticosteroids, because withdrawal from all drugs of abuse studied to date leads to an activated HPA stress response. Interestingly, glucocorticoids, effectors of the HPA-axis, can activate and sensitize CRF-CRF1 systems of the extended amygdala, causally linking the neuroendocrine and extrahypothalamic CRF system stress responses. Consistent with a functional role for the HPA-axis component of the opponent process, glucocorticoid receptor antagonists can reduce the development and expression of excessive alcohol self-administration that results from repeated, intermittent ethanol intoxication (Vendruscolo et al., 2012).
Of preclinical relevance, systemic injections of small molecule CRF1 receptor antagonists that block pituitary and brain CRF1 receptors can also reduce the heightened anxiety-like behavior in dependent rodents acutely withdrawn from alcohol at doses that do not alter the anxiety-like behavior of non-dependent animals (Knapp et al., 2004;Overstreet et al., 2004;Breese et al., 2005a;Breese et al., 2005b;Gehlert et al., 2007;Sommer et al., 2008). Similarly, withdrawal from the repeated administration of cocaine, nicotine, cannabinoids, opiates, and benzodiazepines produces an anxiogenic-like response that can be reversed by intracranial administration of non-selective peptide CRF receptor antagonists (Sarnyai et al., 1995;Rodriguez de Fonseca et al., 1997;Basso et al., 1999;Tucci et al., 2003) or systemic administration of brain-penetrant CRF1-selective nonpeptide receptor antagonists (George et al., 2007;Skelton et al., 2007;Park et al., 2013). Furthermore, the aversive state of opiate withdrawal and the decreased brain reward function associated with nicotine withdrawal are both CRF1 receptor-dependent (Contarino and Papaleo, 2005;Stinus et al., 2005;Bruijnzeel et al., 2007;Bruijnzeel et al., 2009;Bruijnzeel et al., 2012;Garcia-Carmona et al., 2012). Likewise, intracerebroventricular administration of a nonselective CRF1/2 antagonist ameliorated the decreased brain reward function resulting from ethanol withdrawal (Bruijnzeel et al., 2010). Supporting the motivational significance of these effects for addiction, systemic injections of small-molecule CRF1 antagonists reduced the increased alcohol intake of dependent or postdependent rodents (Sabino et al., 2006;Chu et al., 2007;Funk et al., 2007;Gehlert et al., 2007;Gilpin et al., 2008;Richardson et al., 2008) as well as the increased intravenous self-administration of cocaine (Specio et al., 2008), nicotine (George et al., 2007), and heroin (Greenwell et al., 2009) in rats with a history of extended access to the drug of abuse. Similarly, both global (Chu et al., 2007) and conditional brain-specific Crhr1 knockout (Crhr1[NestinCre]) mice (Molander et al., 2012) show reduced ethanol intake during withdrawal in the postdependent state compared with their wildtype littermates.
CRF1 receptor knockout mice also drink less 20% v/v ethanol under basal conditions (Pastor et al., 2011). Moreover, both CRF and CRF1 knockout mice show reduced ethanol intake and blood ethanol concentrations in a murine model of scheduled, limited access to ethanol (“drinking-in-the-dark”) that can produce binge-like intake (Kaur et al., 2012), suggesting an early role for CRF in neuroadaptations associated with the binge/intoxication stage of the addiction cycle. Perhaps accordingly, systemic administration of small-molecule CRF1 antagonists can reduce binge-like but not non-binge-like ethanol intake in C57BL/6J mice and outbred rats (Lowery et al., 2010;Cippitelli et al., 2012;Simms et al., 2013) (but see (Giardino and Ryabinin, 2013) for additional findings suggesting that these effects may not be specific for ethanol). Site-specific infusion of CRF1 antagonists into the CeA or VTA likewise could reduce heightened ethanol intake under intermittent access schedules (Lowery-Gionta et al., 2012;Hwa et al., 2013).
Many individuals who suffer from symptoms of anxiety or depression may turn to a substance of abuse for its potential anxiolytic (e.g., alcohol) or mood-enhancing (e.g., cocaine) effects (Pohorecky, 1991). By reducing dysphoria, CRF1 receptor antagonists may help treat individuals who “self-medicate” their anxious or depressed state with a drug of abuse. Consistent with this hypothesis, small-molecule CRF1 receptor antagonists reduce alcohol drinking in rodent models with high innate anxiety, including genetically selected Marchigian Sardinian alcohol-preferring rats (Ciccocioppo et al., 2006;Hansson et al., 2006;Hansson et al., 2007;Heilig and Koob, 2007;Sommer et al., 2008) and isolation-reared Fawn-hooded rats (Lodge and Lawrence, 2003) at doses that do not alter the intake of normal, outbred rodents.
We and others also found evidence that addiction-like activation of CRF systems may play a role in the motivational properties of palatable food. Specifically, rats acutely withdrawn from intermittent access to a high-sucrose, chocolate-flavored diet showed increased anxiety-like behavior (Cottone et al., 2009). As has been seen with substances of abuse, the increased anxiety-like behavior was accompanied by increased mRNA and peptide expression of CRF in the CeA; similar molecular changes were seen by Bale and colleagues in mice withdrawn from high-fat diet (Teegarden et al., 2009). Systemic pretreatment with the selective CRF1 antagonist R121919 blocked food withdrawal-associated anxiety at doses that did not alter the behavior of chow-fed controls. CRF1 antagonist pretreatment also decreased the magnitude of overeating of the palatable sucrose-rich diet by diet-cycled animals at doses that did not alter the food intake of chow-fed controls or of animals fed the sucrose-rich diet, but without a history of diet cycling. Moreover, R121919 reduced evoked inhibitory postsynaptic potentials in the CeA more in diet-cycled rats than in chow-fed controls, suggesting greater control over CeA GABAergic neurotransmission by CRF1 receptors. The findings resemble the enhanced modulatory influence of CRF1 antagonists on CeA GABAergic synaptic transmission that is seen during withdrawal from alcohol (Roberto et al., 2010). When diet-cycled animals had access to the preferred, sucrose-rich diet, both their anxiety-like behavior and CeA CRF levels normalized, supporting the hypothesis that activation of the amygdala CRF-CRF1 system helped subserve the palatable food withdrawal-like state.
Protracted withdrawal
Symptoms of negative affect can persist for weeks and months after detoxification from drugs of abuse (Alling et al., 1982). These negative emotional symptoms of protracted withdrawal, including anger, frustration, sadness, anxiety and guilt, are subacute and appear to be key precipitants of relapse (Hershon, 1977;Lowman et al., 1996;Zywiak et al., 1996;Annis et al., 1998) in the preoccupation/anticipation stage of addiction. Neuroadaptations in amygdala CRF1 systems have been proposed to promote such protracted abstinence syndromes. Consistent with this hypothesis, increased levels of CRF and the CRF1 receptor have been reported weeks after detoxification from repeated cycles of alcohol intoxication/withdrawal in animal models (Zorrilla et al., 2001;Sommer et al., 2008), and CRF1 receptor antagonists reduce the potentiated anxiogenic-like and ethanol intake behavior responses to otherwise ineffectual stressors seen during protracted withdrawal (Rimondini et al., 2002;Valdez et al., 2002b;Valdez et al., 2003b;Sommer et al., 2008). CRF1 antagonists also attenuate the increased spontaneous anxietylike behavior (Overstreet et al., 2002;Valdez et al., 2002b;Breese et al., 2005a;Breese et al., 2005b;Zhao et al., 2007b;Sommer et al., 2008) and alcohol intake that can be seen in postdependent rats even under low exteroceptive stress conditions (Rimondini et al., 2002;Valdez et al., 2002b;Sommer et al., 2008). Both sets of findings are significant because the resurgence of negative emotional states during protracted withdrawal is a major predictor of relapse in alcoholics (Mossberg et al., 1985;Pickens et al., 1985).
Stress-induced reinstatement
Exposure to external stressors can also increase drug craving (Childress et al., 1994;Cooney et al., 1997;Sinha et al., 2000) and lead to relapse. This stress-induced relapse is hypothesized to be motivated by self-medication of the associated negative emotional symptoms of stress (Lowman et al., 1996;Zywiak et al., 1996), in a fashion similar to self-medication of symptoms of protracted withdrawal, but here elicited by external stimuli. Consistent with this hypothesis, systemic injection of yohimbine, an α2 adrenoceptor antagonist that induces stress-and anxiety-like responses (Bremner et al., 1996), can induce alcohol and heroin craving in human drug addicts (Stine et al., 2002;Umhau et al., 2011). Moreover, yohimbine can reinstate drug-seeking behavior in rats (Shepard et al., 2004;Feltenstein et al., 2012) and monkeys (Lee et al., 2004), as well as the seeking of alcohol (Marinelli et al., 2007) or palatable food in rats (Ghitza et al., 2006). Supporting a role for CRF systems in this relapse mechanism, yohimbine-induced reinstatement of substance-seeking can be prevented by systemic pretreatment with brain-penetrant CRF1 receptor antagonists or intracranial pretreatment with peptide CRF receptor antagonists (Le et al., 2002;Marinelli et al., 2007;Shalev et al., 2010).
Likewise, CRF1 antagonists can reduce stressor-induced reinstatement of substance-seeking in animal models, suggesting a key role for CRF in the preoccupation/anticipation stage of the addiction cycle. For example, systemic injections of CP154,526 attenuated footshock-induced reinstatement of alcohol seeking in nondependent rats (Le et al., 2000b), and subsequent studies showed that antalarmin and MTIP, brain-penetrant nonpeptide CRF1 antagonists, likewise attenuated footshock-induced reinstatement of alcohol seeking, particularly in alcohol-dependent rats and genetically selected, Marchigian Sardinian alcohol-preferring rats (Gehlert et al., 2007), each of which show increased activity of extended amygdala CRF systems (Hansson et al., 2006;Francesconi et al., 2009;Roberto et al., 2010). Mixed CRF1/CRF2 antagonists injected intracranially into the extended amygdala, median raphe, and VTA (see below for details) and small-molecule CRF1 antagonists administered systemically also blocked stressor-induced reinstatement of cocaine-, opiate-, nicotine-, and methamphetamine-seeking behavior (Shaham et al., 1998;Le et al., 2000a;Lu et al., 2003;Shaham et al., 2003;Bruijnzeel et al., 2009;Nawata et al., 2012). A CRF1 antagonist also reduced social defeat stress-induced locomotor sensitization to cocaine and escalated “binge” operant self-administration of cocaine (Boyson et al., 2011) as well as stress-induced reinstatement of cocaine-seeking behavior in rats with a history of extended access to cocaine (Blacktop et al., 2011). A selective CRF1 antagonist reduced shock-induced reinstatement of nicotine-seeking behavior (Plaza-Zabala et al., 2010). Finally, CRF1 knockout mice were resistant to the ability of repeated forced swim stress to increase ethanol intake following deprivation as compared to wildtype mice (Pastor et al., 2011), and conditional brain-specific Crhr1 knockout (Crhr1[NestinCre]) mice were partly resistant to the ability of social defeat stress and forced swim stress to increase ethanol intake (Molander et al., 2012).
CRF1 antagonists are ineffective in blocking cue-, substance-, and context-induced reinstatement, however, indicating specificity of their actions on the stress component of the addiction cycle. This specificity of action is consistent with the unique neuroanatomical and neuropharmacological bases for stress-induced reinstatement as contrasted from overlapping, but distinct, neurocircuits that differentially subserve drug prime- or drug cue- (discrete, discriminative or contextual) induced reinstatement (see (Koob, 2008;Steketee and Kalivas, 2011;Bossert et al., 2013) for reviews).
Data indicate that stressor-induced reinstatement is not mediated by activation of the HPA axis. For example, adrenalectomy did not alter fooshock-induced reinstatement (Le et al., 2000b), and antalarmin had no effect on yohimbine-induced corticosterone secretion at doses that reduced “relapse” behavior (Marinelli et al., 2007). Rather, CRF1 receptor antagonists can reduce stress-induced reinstatement via extrahypothalamic brain sites, as has been shown for alcohol (Le et al., 2000b), cocaine (Erb et al., 1998), heroin (Shaham et al., 1997), nicotine (Zislis et al., 2007), and methamphetamine (Nawata et al., 2012). For example, site-specific blockade of CRF receptors in the median raphe nucleus (Le et al., 2002;Le et al., 2013) was sufficient to attenuate footshock- and yohimbine-induced reinstatement of alcohol seeking. Likewise, intracerebral injections of a mixed CRF1/CRF2 antagonist or a small-molecule CRF1 antagonist into the bed nucleus of the stria terminalis or VTA, but not the amygdala or nucleus accumbens (NAc), reduced reinstatement of substance-seeking behavior (Lu et al., 2003;Shaham et al., 2003). The results agree with other evidence that activation of extrahypothalamic CRF sites subserves stress-induced reinstatement behavior (Shaham et al., 1997;Erb et al., 1998;Wang et al., 2005;Wang et al., 2006;Blacktop et al., 2011). Consistent with this hypothesis, intra-VTA (Wang et al., 2005) or intracerebroventricular (Blacktop et al., 2011;Kupferschmidt et al., 2011;Brown et al., 2012;Buffalari et al., 2012;Kupferschmidt et al., 2012) infusion of CRF can reinstate cocaine-seeking behavior in rats.
Some evidence supports a role for NAc CRF1 systems in promoting substance consumption, if not seeking, during stress. For example, restraint stress during deprivation from ethanol can stimulate increased ethanol intake upon renewed access in ethanol-preferring P rats, an effect that can be blocked by intra-NAc, but not intra-amygdala, intra-dorsal raphe, or intra-VTA, administration of a CRF1 antagonist (Knapp et al., 2011). Consistent with above, CRF injection into the NAc, but not VTA, raphe, or amygdala, can potentiate ethanol intake following a deprivation period (Knapp et al., 2011). More research is needed to determine conditions under and mechanisms via which CRF1 systems of the VTA and NAc may differentially influence reinstatement of seeking vs. consummatory behavior, respectively.
Corticotropin-releasing factor, stress, and the frontal cortex
Converging evidence may link the impairment of medial prefrontal cortex (mPFC) cognitive function, activation of CRF in the PFC, and overactivation of the CeA with the development of compulsive-like responding for drugs of abuse, again suggesting a role for CRF in the binge/intoxication stage of the addiction cycle (Briand et al., 2008a;Briand et al., 2008b;George et al., 2008). Extended access to drugs of abuse, such as cocaine self-administration, can induces a compulsive-like pattern of intake taking that associates with impaired working memory (George et al., 2008). Whereas long-access (LgA) and short-access (ShA) rats both exhibit mostly correct responses in a delayed non-matching-to-sample task under low cognitive demand (delay < 10 s), working memory performance of LgA rats is substantially impaired by increasing the delay. The magnitude of escalation of cocaine intake correlates directly with the impaired working memory performance at the long delay. Furthermore, deficits in working memory were accompanied by a decreased density of dorsomedial PFC (dmPFC) neurons or oligodendrocytes that persisted for months despite cocaine cessation. Thus, dmPFC dysfunction might contribute to the loss of control associated with compulsive drug use and facilitate the progression to drug addiction.
CRF may be implicated in the reviewed frontal cortex dysfunction. Rats receiving chronic intermittent access to two-bottle choice alcohol drinking, a paradigm that leads to escalated alcohol intake (Wise, 1973;Simms et al., 2008), show increased activation of GABA and CRF neurons in the mPFC during abstinence. Working memory impairments in these rats correlate directly with greater alcohol drinking during acute abstinence (George et al., 2012). Abstinence was also associated with a functional disconnection of the mPFC and CeA, but not hippocampus or NAc. The results show a recruitment of a subset of GABA and CRF neurons in the mPFC during alcohol withdrawal and suggest that disconnection of the PFC-CeA pathway may be key to impaired executive control over motivated behavior. In summary, dysregulation of mPFC interneurons may be an early index of the transition to alcohol dependence.
Clinical trials of CRF1 receptor antagonists in addiction
Collectively, the reviewed studies demonstrate a key role for brain CRF1 receptors in three addiction-related domains: (1) negative emotional symptoms of acute and protracted withdrawal that can occur sans exteroceptive stress, (2) escalated, compulsive-like substance intake (e.g., with substance dependence), and (3) stress-induced relapse to substance seeking. Accordingly, small-molecule CRF1 antagonists are currently in clinical trials for stress-related aspects of the addiction process. GlaxoSmithKline and the National Institutes of Health (NIH) are evaluating whether verucerfont can reduce stress-induced alcohol craving in anxious, stress-reactive alcoholic women (NCT01187511). Bristol Myers Squibb and NIH are testing whether pexacerfont can prevent stress-induced craving for palatable food in dieters (NCT01656577), stress-induced craving for tobacco in abstaining smokers (NCT01557556), and stress-induced craving for alcohol in anxious alcoholic women (NCT01227980) (Zorrilla et al., 2013a).
Functional and genetic heterogeneity of substance use disorders
The effectiveness of medications for substance use disorders differs across individuals and even within individuals at different times of their disease process (Heilig et al., 2010b;Koob and Zorrilla, 2010;Heilig et al., 2011;Logrip et al., 2011;Logrip et al., 2012). Based on the reviewed evidence, CRF1 receptor antagonists would be expected to be most effective if substance use has transitioned to use driven by negative reinforcement (withdrawal/negative affect) and to protect against stress-induced relapse (stress-related craving). On the other hand, CRF1 antagonists would be predicted to less effectively reduce reward-motivated, recreational substance abuse earlier in the addiction process (Heilig and Koob, 2007) or to mitigate relapse episodes that were precipitated by cues or contexts associated with previous drug taking (Liu and Weiss, 2002).
Given that substance use disorders are partly heritable, pharmacogenetic differences also may be relevant to CRF1 antagonist pharmacotherapy, as is true of other treatments (Heilig et al., 2011;Sinha, 2011). Indeed, animal models support the hypothesis that gene variants for CRF system molecules may promote negatively-reinforced alcohol intake. For example, many msP alcohol-preferring rats carry two G-to-A polymorphisms in allelic identity with one another in the distal promoter of the Crhr1 gene. These mutations are not seen in other alcohol-preferring lines or outbred rats (Hansson et al., 2006; Logrip, Walker, Ayanwuyi, Sabino, Ciccocioppo, Walker, Koob and Zorrilla, unpublished observations). Perhaps as a result, the msP line exhibits increased CRF1 receptor expression in several stress-related brain regions, increased anxiety-like behavior, and increased sensitivity to the ability of CRF1 receptor antagonists to reduce alcohol self-administration and stress-induced reinstatement of alcohol seeking (Ciccocioppo et al., 2006;Hansson et al., 2006;Gehlert et al., 2007;Ayanwuyi et al., 2013; Logrip, Walker, Ayanwuyi, Sabino, Ciccocioppo, Walker, Koob and Zorrilla, unpublished observations). Similarly, rhesus monkeys that carry a C-to-T single nucleotide polymorphism in the promoter of the Crh gene do not show normal glucocorticoid feedback inhibition of CRF peptide expression. This gene variant is associated with two-fold greater alcohol consumption in monkeys exposed to early life stress, without altering the basal drinking of unstressed monkeys (Barr et al., 2009).
Several polymorphisms in human CRF system molecules have also been associated with alcohol use phenotypes, often in interaction with stress history. Several Crhr1 haplotype variants in adolescents predict binge drinking and lifetime prevalence of intoxication and alcohol dependence (Treutlein et al., 2006). Crhr1 single-nucleotide polymorphisms (SNPs) also predicted greater alcohol consumption in already dependent individuals (Treutlein et al., 2006). A study of 1,049 Caucasian subjects from 209 families in the Collaborative Study on the Genetics of Alcoholism (COGA) also found significant associations between the P3 amplitude and alcohol dependence with multiple SNPs in the Crhr1 gene (Chen et al., 2010). Stress history produces greater increases in future alcohol intake (Blomeyer et al., 2008;Schmid et al., 2010) and an earlier onset of drinking (Schmid et al., 2010) in adolescents homozygous for the C allele of the rs1876831 SNP of the Crhr1 gene. Adolescent carriers of the A allele of the rs242938 Crhr1 SNP similarly reported more drinking when exposed to stress in some (Schmid et al., 2010) but not other (Blomeyer et al., 2008) studies. Conversely, adolescents homozygous for the H2 haplotype containing the rs1876831 minor allele are protected against early abuse-associated increases in alcohol intake and dependence (Nelson et al., 2010).
CRF system polymorphisms in the CRF binding protein (CRF-BP), which the Vale laboratory discovered to moderate the ability of CRF to interact with its receptors (Potter et al., 1991;Potter et al., 1992;Behan et al., 1993;Behan et al., 1995), also have been linked to human alcohol phenotypes. For example, Crhbp gene SNPs are associated with an endophenotype of alcoholism (i.e., decreased electroencephalographic alpha wave power) and are more prevalent in alcohol use disorders (Enoch et al., 2008), including in alcoholics with comorbid anxiety disorders (Enoch et al., 1999). A Crhbp polymorphism (rs10055255) has also been associated with greater stress imagery-induced alcohol craving and dysphoria (Ray, 2011). Furthermore, SNPs in the Crhbp (rs3811939) and Crhr1 (the widely studied rs110402 polymorphism) genes jointly predicted comorbid alcohol use disorder in patients with schizophrenia (Ribbe et al., 2011), with greater ratios of mononuclear Crhr1/Crhbp mRNA seen in dual carriers of the polymorphism (Ribbe et al., 2011). Finally, a recent prospective study showed that CRF-BP genotype moderated the relationship between stress-induced negative affect and the self-reported negative consequences of drinking (Tartter and Ray, 2012). Further work is needed to determine whether similar gene variants are associated with phenotypes for other substance use disorders. Perhaps pharmacogenomic profiling could identify patients for whom CRF1 receptor antagonist pharmacotherapy would be especially useful to prevent relapse (Sinha, 2011).
CRF2 systems and addiction
Whereas CRF1 systems are generally recognized to exert an overarching, pro-stress-like effect, CRF2 receptor activation may, in addition to suppressing food intake (Spina et al., 1996;Inoue et al., 2003;Fekete et al., 2007), decrease stress responsiveness (Valdez et al., 2002a;Valdez et al., 2003a). In the context of addiction-related behavior, intracerebroventricular infusion of Ucn 3, a selective CRF2 agonist, reduced the heightened anxiety-like behavior and increased ethanol self-administration in dependent rats during acute withdrawal (Valdez et al., 2004) as well as the alcohol intake of mice that receive limited, binge-like access to alcohol (Sharpe and Phillips, 2009;Lowery et al., 2010). Site-specific infusion of Ucn 3 into the CeA likewise reduced the heightened alcohol self-administration in withdrawn, dependent rats (Funk and Koob, 2007), and microinjection of Ucn 1 into the CRF2 receptor-rich lateral septum potently reduced the acquisition and expression of alcohol intake in rats (Ryabinin et al., 2008). There is controversy in this area, however, because pharmacological activation of CRF2 receptors has been found to decrease anxiety-like behavior, increase anxiety-like behavior, or not alter stress-related behavior, depending on the neuropharmacological probe, dose, and brain site (Ho et al., 2001;Takahashi et al., 2001;Fekete and Zorrilla, 2007;Zhao et al., 2007a). Accordingly, (any) influence of the CRF2 system on addiction-related behavior may also be brain-region specific.
CRF2 receptors in stress-induced reinstatement
CRF2 receptors outside the extended amygdala have been suggested to act in concert with CRF1 receptors to facilitate aspects of compulsive-like drug seeking. VTA CRF2 receptors have been proposed to facilitate stress-induced reinstatement of cocaine seeking, in which footshock exposure in cocaine-experienced rats elicited the release of extended amygdala-derived CRF into the VTA (Wang et al., 2005). The CRF-mediated stress-induced reinstatement of cocaine seeking is putatively mediated by the sensitization of VTA glutamate release. The activation of VTA CRF2, rather than CRF1, receptors, was proposed to mediate these actions (Wang et al., 2007), but it should be noted that the putative CRF2 antagonist used, anti-sauvagine-30, is not highly selective for CRF2 receptors (Zorrilla et al., 2013b). Indeed, others who used the more selective CRF2 antagonist astressin2-B did not observe a reversal of stress-induced reinstatement of substance seeking (Bruijnzeel et al., 2009), and negligible CRF2 receptor mRNA is detected in the rodent VTA by in situ hybridization under basal conditions (Chalmers et al., 1995;Van Pett et al., 2000). Nevertheless, the possible role for CRF2 receptors in stress-induced relapse to drug seeking also implicates a possible role for the CRF peptide family members that have high affinity for CRF2 receptors (Gysling, 2012), including Ucn 1, 2 and 3.
CRF2 receptor and opioid withdrawal
Recent studies have obtained evidence that the CRF2 receptor may play a key role in opiate withdrawal. First, whereas knockout of the CRF1 receptor exacerbated the somatic signs of opiate withdrawal (Papaleo et al., 2007), genetic deletion of the CRF2 receptor blocked the somatic signs of opiate withdrawal (Papaleo et al., 2008). Moreover, CRF2 knockout mice did not manifest the dysphoria-like or anhedonia-like behaviors of opiate withdrawal (Ingallinesi et al., 2012), whereas they showed normal neuroendocrine responses, as indexed by HPA activation (e.g., CRF in the paraventricular nucleus the hypothalamus). The findings potentially implicate CRF2 receptors in driving extrahypothalamic CRF and dynorphin responses that subserve opiate withdrawal distress, perhaps via presynaptic positive regulation of CRF synthesis and release (Ingallinesi et al., 2012).
Urocortin 1 and alcohol intake
In the brain, Ucn 1, which has equal activity at CRF1 vs. CRF2 receptors, is synthesized principally in cell bodies of the stress-responsive (Korosi et al., 2005) perioculomotor Ucn 1-containing area (pIIIu), now also known as the nonpreganglionic Edinger-Westphal nucleus (Weitemier et al., 2005), and, to a lesser extent, in the lateral superior olive (Vaughan et al., 1995;Ryabinin et al., 2005). Ucn 1 was first implicated in alcohol consumption based on the preferential induction of Fos expression in Ucn 1-containing neurons (Bachtell et al., 2002;Ryabinin et al., 2003;Spangler et al., 2009) of the pIIIu following voluntary alcohol drinking in multiple species (Topple et al., 1998;Ryabinin et al., 2001;Weitemier et al., 2001;Bachtell et al., 2003;Sharpe et al., 2005;Turek and Ryabinin, 2005;Kaur and Ryabinin, 2010;Anacker et al., 2011) and because basal Ucn1 immunoreactivity in the pIIIu is elevated in several inbred or selectively bred rodent lines that demonstrate high alcohol drinking phenotypes (Bachtell et al., 2002;Bachtell et al., 2003;Turek and Ryabinin, 2005;Fonareva et al., 2009) or that show increased sensitivity to the rewarding, hypothermic, or sedative actions of ethanol (Bachtell et al., 1999;Kiianmaa et al., 2003;Ryabinin and Weitemier, 2006;Turek et al., 2008). Accordingly, electrolytic lesions of the centrally projecting Edinger-Westphal nucleus in C57BL/6J mice attenuated ethanol intake and preference in a two-bottle choice paradigm without influencing the consumption of sucrose, quinine, saccharin, or saline (Bachtell et al., 2004;Weitemier and Ryabinin, 2005). Consistent with a role for Edinger-Westphal Ucn 1 in ethanol preference, either electrolytic lesion of the Edinger Westphal nucleus or Ucn 1 knockout reduces ethanol preference, but neither treatment produces an additional effect above and beyond the other (Giardino et al., 2011). Furthermore, ethanol did not promote a conditioned place preference in mice deficient in Ucn 1 or the CRF2 receptor (Giardino et al., 2011). The reviewed results support the hypothesis that centrally projecting Ucn 1 neurons from the Edinger-Westphal nucleus may promote ethanol intake, preference, or reward, perhaps via actions on CRF2 receptors. Limiting this conclusion, however, bilateral administration of Ucn 1 into the mouse lateral septum selectively attenuated alcohol self-administration during both the acquisition and expression of a limited access alcohol drinking procedure (Ryabinin et al., 2008). More work is needed to elucidate which specific Ucn 1-containing circuits promote vs. oppose ethanol use and the applicability of reviewed findings to other substances of abuse.
Concluding remarks
On a personal note, Wylie Vale was the impetus and driving force behind our early work on the role of CRF in behavioral responses to stressors. We (Floyd Bloom, George Siggins, and myself, George Koob) joined Vale on the NIH program project grant from the National Institute on Diabetes and Digestive and Kidney Diseases when CRF was discovered in 1981. We subsequently injected CRF into the brain of rats and charted their behavioral responses in collaboration with Vale and colleagues (Sutton et al., 1982). The resulting behavioral profile and subsequent studies led to the overall hypothesis that CRF, in addition to its effects on the HPA axis, also mediated behavioral responses to stressors. This work proceeded in parallel, but independent of, our addiction work until we conceptualized between-system neuroadaptations as explanations for opponent process (Koob and Bloom, 1988) and invoked the hypothesis that activation of the brain CRF systems was a key player in such between-system neuroadaptations. During this period, as data implicating the role for CRF in addiction consolidated, Eric Zorrilla joined our joint Salk/Scripps project extending our work to protracted abstinence and compulsive eating.
Wylie Vale was the guiding light to our work conceptually, innovatively, and motivationally. He embraced our overall hypothesis that CRF drove the “dark side” of addiction, provided the molecular advances and tools to test this hypothesis (knockout mice, discovery of the CRF1 and CRF2 receptors, powerful antibodies, discovery of urocortins, etc.), and delighted us with his constant enthusiasm for each new finding. Wylie Vale was a force of nature who had a tremendous impact on the fields of neuroendocrinology and neuroscience because he embraced any reasonable hypothesis that was supported by the data and let the work on CRF be guided not by preconceived notions. Not to mention his wonderful humor, incredible curiosity and intellect. We miss him terribly.
Highlights.
CRF systems have emerged as mediators of the body’s response to stress and, relatedly, the pathophysiology of addiction.
CRF systems have a prominent role in driving the addiction via actions in the central extended amygdala.
Addiction-related actions include anxious behavior, brain reward deficits, excessive drug use and stress-induced drug-seeking.
Polymorphisms in CRF system molecules are associated with drug use phenotypes in humans, often with stress history.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alling C, Balldin J, Bokstrom K, Gottfries CG, Karlsson I, Langstrom G. Studies on duration of a late recovery period after chronic abuse of ethanol. A cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr Scand. 1982;66:384–397. doi: 10.1111/j.1600-0447.1982.tb06720.x. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis HM, Sklar SM, Moser AE. Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics. Addict Behav. 1998;23:127–131. doi: 10.1016/s0306-4603(97)00024-5. [DOI] [PubMed] [Google Scholar]
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front Psychiatry. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxiety-like behavior: molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett. 2011;490:220–225. doi: 10.1016/j.neulet.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the "anxiogenic-like" effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Behan DP, Potter E, Lewis KA, Jenkins NA, Copeland N, Lowry PJ, Vale WW. Cloning and structure of the human corticotrophin releasing factor-binding protein gene (CRHBP) Genomics. 1993;16:63–68. doi: 10.1006/geno.1993.1141. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005a;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II.clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008a;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gross JP, Robinson TE. Impaired object recognition following prolonged withdrawal from extended-access cocaine self-administration. Neuroscience. 2008b;155:1–6. doi: 10.1016/j.neuroscience.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Kupferschmidt DA, Erb S. Reinstatement of cocaine seeking in rats by the pharmacological stressors, corticotropin-releasing factor and yohimbine: role for D1/5 dopamine receptors. Psychopharmacology (Berl) 2012;224:431–440. doi: 10.1007/s00213-012-2772-3. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav. 2012;101:62–68. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Small E, Pasek TM, Yamada H. Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behav Brain Res. 2010;210:288–291. doi: 10.1016/j.bbr.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;105:209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Manz N, Tang Y, Rangaswamy M, Almasy L, Kuperman S, Nurnberger J, Jr, O'connor SJ, Edenberg HJ, Schuckit MA, Tischfield J, Foroud T, Bierut LJ, Rohrbaugh J, Rice JP, Goate A, Hesselbrock V, Porjesz B. Single-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Mclellan AT, Macrae J, Natale M, O'brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacol Biochem Behav. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, Albaugh B, Hodgkinson CA, Goldman D. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3:e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin Exp Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res. 2009;33:1956–1965. doi: 10.1111/j.1530-0277.2009.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O'dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O'dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carmona JA, Almela P, Baroja-Mazo A, Milanes MV, Laorden ML. Restricted role of CRF1 receptor for the activity of brainstem catecholaminergic neurons in the negative state of morphine withdrawal. Psychopharmacology (Berl) 2012;220:379–393. doi: 10.1007/s00213-011-2478-y. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, Mckinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS One. 2011;6:e26997. doi: 10.1371/journal.pone.0026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 Receptor Signaling Regulates Food and Fluid Intake in the Drinking-in-the-Dark Model of Binge Alcohol Consumption. Alcohol Clin Exp Res. 2013;37:1161–1170. doi: 10.1111/acer.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol Pharmacol. 1996;50:679–686. [PubMed] [Google Scholar]
- Gysling K. Relevance of both type-1 and type-2 corticotropin releasing factor receptors in stress-induced relapse to cocaine seeking behaviour. Biochem Pharmacol. 2012;83:1–5. doi: 10.1016/j.bcp.2011.07.101. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010a;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010b;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Debold JF, Miczek KA. Alcohol in excess: CRF(1) receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl) 2013;225:313–327. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingallinesi M, Rouibi K, Le Moine C, Papaleo F, Contarino A. CRF2 receptor-deficiency eliminates opiate withdrawal distress without impairing stress coping. Mol Psychiatry. 2012;17:1283–1294. doi: 10.1038/mp.2011.119. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Kaur S, Li J, Stenzel-Poore MP, Ryabinin AE. Corticotropin-releasing factor acting on corticotropin-releasing factor receptor type 1 is critical for binge alcohol drinking in mice. Alcohol Clin Exp Res. 2012;36:369–376. doi: 10.1111/j.1530-0277.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianmaa K, Hyytia P, Samson HH, Engel JA, Svensson L, Soderpalm B, Larsson A, Colombo G, Vacca G, Finn DA, Bachtell RK, Ryabinin AE. New neuronal networks involved in ethanol reinforcement. Alcohol Clin Exp Res. 2003;27:209–219. doi: 10.1097/01.ALC.0000051020.55829.41. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Huang M, Wills TA, Whitman BA, Angel RA, Sinnett SE, Breese GR. Effects of a stressor and corticotrophin releasing factor on ethanol deprivation-induced ethanol intake and anxiety-like behavior in alcohol-preferring P rats. Psychopharmacology (Berl) 2011;218:179–189. doi: 10.1007/s00213-011-2366-5. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Klas PG, Erb S. Cannabinoid CB1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine-induced behavioural sensitization. Br J Pharmacol. 2012;167:196–206. doi: 10.1111/j.1476-5381.2012.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Lovejoy DA, Rotzinger S, Erb S. Teneurin C-terminal associated peptide-1 blocks the effects of corticotropin-releasing factor on reinstatement of cocaine seeking and on cocaine-induced behavioural sensitization. Br J Pharmacol. 2011;162:574–583. doi: 10.1111/j.1476-5381.2010.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2013;18:448–451. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe in relapse to alcohol seeking in rats. J. Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000a;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000b;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003;117:243–247. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;25:271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2012;62:552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt's taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction. 1996;91(Suppl):S51–S71. [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez De Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossberg D, Liljeberg P, Borg S. Clinical conditions in alcoholics during long-term abstinence: a descriptive, longitudinal treatment study. Alcohol. 1985;2:551–553. doi: 10.1016/0741-8329(85)90133-8. [DOI] [PubMed] [Google Scholar]
- Nawata Y, Kitaichi K, Yamamoto T. Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock. Pharmacol Biochem Behav. 2012;101:297–302. doi: 10.1016/j.pbb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict Biol. 2010;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Ghozland S, Ingallinesi M, Roberts AJ, Koob GF, Contarino A. Disruption of the CRF(2) receptor pathway decreases the somatic expression of opiate withdrawal. Neuropsychopharmacology. 2008;33:2878–2887. doi: 10.1038/npp.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Kitchener P, Contarino A. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007;53:577–589. doi: 10.1016/j.neuron.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF. Corticotropin-releasing factor (CRF) and alpha 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol. 2013:1–9. doi: 10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol Behav. 2011;104:149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, Stenzel-Poore MP, Phillips TJ. Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berl) 2011;218:169–177. doi: 10.1007/s00213-011-2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens RW, Hatsukami DK, Spicer JW, Svikis DS. Relapse by alcohol abusers. Alcohol Clin Exp Res. 1985;9:244–247. doi: 10.1111/j.1530-0277.1985.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, De Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin Exp Res. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, Sperling S, Friedrichs H, Jahn O, Sillaber I, Gefeller O, Krampe H, Ehrenreich H. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch Gen Psychiatry. 2011;68:1247–1256. doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Freeman P, Risinger FO. ITF expression in mouse brain during acquisition of alcohol self-administration. Brain Res. 2001;890:192–195. doi: 10.1016/s0006-8993(00)03251-0. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;134:1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151:780–790. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates 'anxiety-like' behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Phillips TJ. Central urocortin 3 administration decreases limited-access ethanol intake in nondependent mice. Behav Pharmacol. 2009;20:346–351. doi: 10.1097/FBP.0b013e32832f01ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Simms JA, Nielsen CK, Li R, Bartlett SE. Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395. Addict Biol. 2013 doi: 10.1111/adb.12024. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Gutman DA, Thrivikraman KV, Nemeroff CB, Owens MJ. The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal. Psychopharmacology (Berl) 2007;192:385–396. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivationITemporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol. Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Tartter MA, Ray LA. A prospective study of stress and alcohol craving in heavy drinkers. Pharmacol Biochem Behav. 2012;101:625–631. doi: 10.1016/j.pbb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, Mcgregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O'dell LE. Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front Psychiatry. 2013;4:38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, alpha-helical CRF(9–41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- Turek VF, Bennett B, Ryabinin AE. Differences in the urocortin 1 system between long-sleep and short-sleep mice. Genes Brain Behav. 2008;7:113–119. doi: 10.1111/j.1601-183X.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George TD, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;60:303–311. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002a;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002b;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003a;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003b;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Et Al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005;119:1235–1243. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Backstrom P, Hyytia P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol Clin Exp Res. 2001;25:704–710. [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2010;34:1603–1612. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007a;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007b;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Heilig M, De Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013a;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Roberts AJ, Rivier JE, Koob GF. Anxiolytic-like effects of antisauvagine-30 in mice are not mediated by CRF2 receptors. PLoS One. 2013b;8:e63942. doi: 10.1371/journal.pone.0063942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF, Weiss F. Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol. 2012;17:300–308. doi: 10.1111/j.1369-1600.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zywiak WH, Connors GJ, Maisto SA, Westerberg VS. Relapse research and the Reasons for Drinking Questionnaire: a factor analysis of Marlatt's relapse taxonomy. Addiction. 1996;91(Suppl):S121–S130. [PubMed] [Google Scholar]