This work identifies the role of ROP3 GTPase in embryo development and auxin-dependent plant growth and shows that ROP3 is important to maintain the proper polarity of auxin efflux carriers PIN1 and PIN3 at the plasma membrane. The results link cell polarity to auxin-dependent patterning through the activity of ROP3.
Abstract
ROP GTPases are crucial for the establishment of cell polarity and for controlling responses to hormones and environmental signals in plants. In this work, we show that ROP3 plays important roles in embryo development and auxin-dependent plant growth. Loss-of-function and dominant-negative (DN) mutations in ROP3 induced a spectrum of similar defects starting with altered cell division patterning during early embryogenesis to postembryonic auxin-regulated growth and developmental responses. These resulted in distorted embryo development, defective organ formation, retarded root gravitropism, and reduced auxin-dependent hypocotyl elongation. Our results showed that the expression of AUXIN RESPONSE FACTOR5/MONOPTEROS and root master regulators PLETHORA1 (PLT1) and PLT2 was reduced in DN-rop3 mutant embryos, accounting for some of the observed patterning defects. ROP3 mutations also altered polar localization of auxin efflux proteins (PINs) at the plasma membrane (PM), thus disrupting auxin maxima in the root. Notably, ROP3 is induced by auxin and prominently detected in root stele cells, an expression pattern similar to those of several stele-enriched PINs. Our results demonstrate that ROP3 is important for maintaining the polarity of PIN proteins at the PM, which in turn ensures polar auxin transport and distribution, thereby controlling plant patterning and auxin-regulated responses.
INTRODUCTION
Plant Rho-like small G proteins called RAC/ROPs (ROPs will be used from now on) function as molecular switches in diverse signaling cascades and mediate leaf cell morphogenesis, polarized cell growth in pollen tubes and root hairs, and hormone and defense-related responses (Nibau et al., 2006; Yang and Fu, 2007; Yalovsky et al., 2008; Wu et al., 2011). ROPs shuttle between a GTP-bound active form and a GDP-bound inactive form. In their active state, ROPs interact with effector proteins to initiate myriad downstream signaling pathways.
The Arabidopsis thaliana genome encodes 11 ROPs. We have previously demonstrated that several members of the Arabidopsis ROP family and RAC1 from tobacco (Nicotiana tabacum) mediate auxin-signaled gene expression and that perturbing the signaling capacity of these small GTPases induces auxin-related developmental defects (Tao et al., 2002, 2005). The precise contribution from individual ROPs to auxin signaling remains largely unknown. Recent studies have indicated the involvement of ROP signaling in the regulation of polar auxin transport, which generates auxin gradients and underlies almost all aspects of auxin controlled growth and developmental responses. ROP2 has been implicated in the regulation of PIN1 endocytosis in the leaf epidermal pavement cells (Nagawa et al., 2012). ROP6, together with its effector RIC1 (ROP-interactive CRIB motif-containing protein1), control clathrin-dependent PIN1/PIN2 endocytosis and mediate root gravitropic responses and leaf vasculature development (Chen et al., 2012; Lin et al., 2012). A ROP effector protein ICR1 (interactor of constitutive active ROP1) is required for embryo development, root meristem organization, and polar localization of PIN1 and PIN2 (Hazak et al., 2010).
Polar auxin transport is mediated by the PIN family of auxin efflux proteins and the AUX1/LAX family of influx carrier proteins (Bennett et al., 1996; Swarup et al., 2008). Genetic elimination of components in auxin transport, e.g., by mutations in members of the PIN proteins, such as PIN1, 3, 4, and 7 (Friml et al., 2003; Blilou et al., 2005), underlies defects in many auxin-regulated processes. Of the PINs, PIN1 is involved in the mediation of auxin accumulation in the base of early globular embryos to initiate hypophysis specification and postembryonic root growth (Petricka et al., 2012). PIN3 is required for auxin flow to the root tip and redistribution within root tissues, which influences tropic responses (Friml et al., 2002; Blilou et al., 2005; Grieneisen et al., 2007). PIN proteins exhibit polar localization at the plasma membrane and determine the direction of intercellular auxin flow (Wisniewska et al., 2006). PIN polar localization and asymmetric auxin distribution are regulated by multiple factors, including regulated endocytosis, recycling, and retromer-dependent vacuolar targeting (Steinmann et al., 1999; Geldner et al., 2003; Jaillais et al., 2007; Kleine-Vehn et al., 2008b). In addition, phosphorylation and dephosphorylation cycles mediated by the AGC kinases PINOID (PID) and PID-related AGC3 and the phosphatase PP2A are important for dynamic changes of PIN polarity. Both PID and PP2A have been shown to be required for auxin transport-related embryo development and postembryonic organogenesis (Christensen et al., 2000; Friml et al., 2004; Michniewicz et al., 2007; Dhonukshe et al., 2010).
How ROPs regulate auxin-dependent development remains largely unknown. RopGEFs are guanine nucleotide exchange factors (GEFs) that stimulate GDP-GTP exchange and activate ROPs (Berken et al., 2005). We showed recently that one of the 14 Arabidopsis ROPGEFs, RopGEF7, is important for embryo and root meristem pattern formation controlled by the auxin/PLETHORA (PLT)-dependent pathway (Chen et al., 2011). We also established that ROP3 interacts directly with RopGEF7 (Chen et al., 2011). Here, we explore the developmental role of ROP3 by analyzing rop3 T-DNA insertion and dominant-negative rop3 mutant lines. Mutant phenotypes show that ROP3 has broad biological roles and is important for the regulation of embryo development, postembryonic organ formation, root gravitropism, hypocotyl elongation, and root growth sensitivity to auxin. During embryogenesis, perturbing ROP3 function reduces PIN1 abundance in the plasma membrane (PM), whereas in the seedling stage, it causes a basal to apical shift of PIN1 and PIN3 localization. Our data indicate that ROP3 acts in polar auxin transport and thus controls the establishment of auxin maxima, which underlies embryo development and seedling growth.
RESULTS
ROP3 Is Expressed in Developing Embryos and Postembryonic Stages
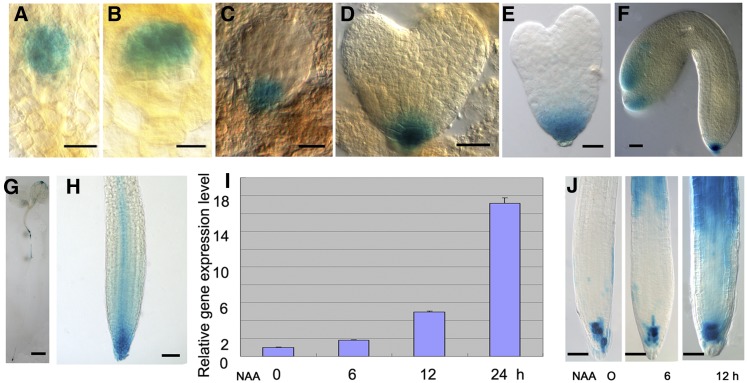
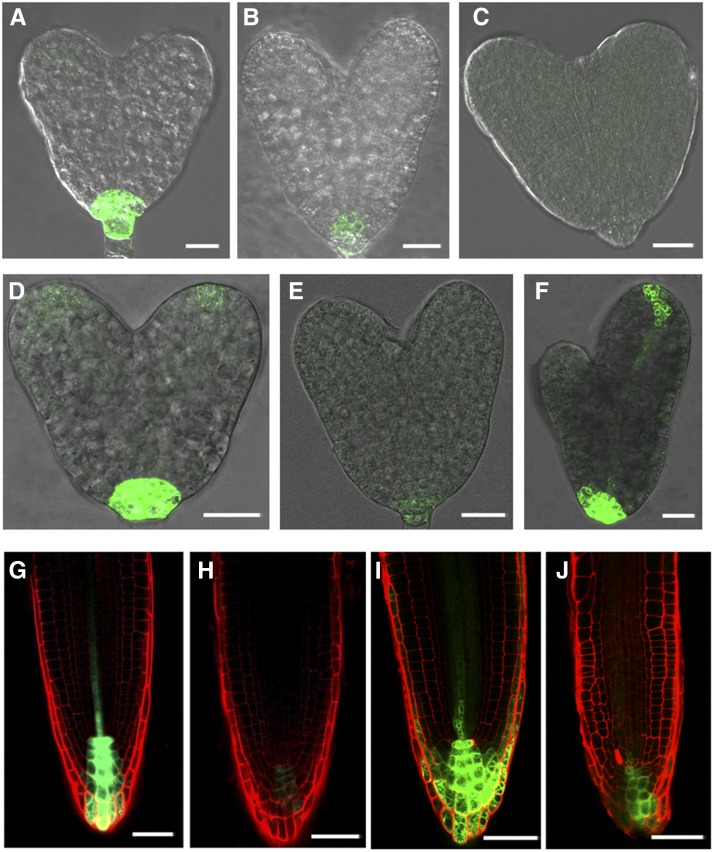
We used a ROP3pro:GUS (β-glucuronidase) gene fusion to study the spatial expression pattern of ROP3. Analysis of GUS activity in ROP3pro:GUS lines revealed that ROP3 was expressed in embryonic and postembryonic stages (Figure 1; Supplemental Figure 1). In globular stage embryos, GUS activity was detected only in the hypophysis (Figure 1C). At the heart and late heart stages, GUS activity was maintained in the daughter cells of the hypophysis (Figures 1D and 1E). During the late stages, GUS activity was also detected in the tip regions of cotyledons in addition to the root pole (Figure 1F). When GUS staining duration was prolonged from 16 to 36 h, GUS activity could be detected in the embryo proper, but not in the hypophysis at the octant (Figure 1A) and 16-cell stages (Figure 1B), indicating that ROP3 is expressed in the earliest stage of embryo development. In the seedling stage, we found GUS signals in the root, hypocotyl, and cotyledons (Figure 1G). Upon closer examination of root tips, the region that showed the GUS staining appeared to be in the quiescent center, columella stem cells, and columella cell layers (Figure 1J, left), and stele cells in root meristem showed relatively weak GUS signal (Figure 1H). ROP3 was also expressed in guard cells of cotyledons (Supplemental Figure 1A) and at a much higher level in the anther and pollen (Supplemental Figures 1B and 1C). The observed ROP3pro:GUS expression profile matches the results of public expression databases (e.g., http://www.ebi.ac.uk/arrayexpress). For instance, the pattern in seedling roots as visualized by GUS reporter gene expression was consistent with microarray results for ROP3 from different root sections (Dinneny et al., 2008; http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi?dataSource=Root). To further validate this observation, we performed quantitative RT-PCR (qRT-PCR) analysis on root sections. The results showed that ROP3 was expressed in root columella and meristem, elongation, and maturation regions (Supplemental Figure 1D). The expression level of ROP3 was relatively higher in the root columella and meristem and elongation zones in contrast to that in the root maturation zone (Supplemental Figure 1D). This result is consistent with data from microarray analysis of root sections (Dinneny et al., 2008).
Figure 1.
Expression of ROP3pro:GUS.
(A) to (F) ROP3pro:GUS is expressed in octant (A), 16-cell (B), globular (C), heart (D), late heart (E), and bent (F) embryo stages.
(G) and (H) ROP3pro:GUS is expressed in a 5-d-old seedling (G) and root tip (H).
(I) qRT-PCR analysis showed that ROP3 expression in roots of 7-d-old wild-type seedlings was enhanced by 10 μM NAA treatment for various times, as indicated.
(J) GUS staining showed that GUS activity in roots of 5-d-old ROP3pro:GUS seedlings is induced by 10 μM NAA for 6 (middle) and 12 (right) h; untreated sample is used as control (left).
GUS staining was for 36 h in (A) and (B), 16 h in (C) to (H), and 6 h in (J). Bars = 10 μm in (A) to (C), 20 μm in (D) to (F), 1mm in (G), and 50 μm in (H) and (J).
We also examined whether ROP3 expression is influenced by auxin. qRT-PCR analysis showed that the expression of ROP3 was induced by auxin in the roots of seedlings. Treatment with 10 μM 1-naphthaleneacetic acid (NAA) for 12 h clearly enhanced the transcript levels of ROP3, reaching the highest level after 24 h of NAA treatment (Figure 1I). Further study using transgenic plants expressing ROP3pro:GUS confirmed that auxin treatment increased the expression of ROP3, which is detectable at the elongation zone of the primary roots (Figure 1J), consistent with ROP3 being involved in auxin-regulated pathways.
We next constructed a fusion to yellow fluorescent protein (YFP) to examine the subcellular localization of ROP3 in root cells (Supplemental Figures 1E to 1H). YFP:ROP3 was predominantly localized in the PM of root cells (Supplemental Figures 1E and 1F). We also examined the subcellular localization of the dominant negative DN-rop3 (see below) and found that in roots, YFP:DN-rop3 showed diminished PM accumulation and was mainly detected in the cytoplasm and the perinuclear region (Supplemental Figures 1G and 1H).
Ectopic Expression of DN-rop3 or Loss of Function in ROP3 Affects Embryo Patterning
Replacement of specific amino acid residues in GTP binding domain in ROPs is a commonly used and informative method to create dominant mutations in these small GTPases (Feig, 1999; Yang, 2002). Based on this, we constructed the dominant-negative (DN) mutant DN-rop3, which presumably locks ROP3 in the inactive form, and explored the functional role of ROP3 in embryo development in DN-rop3 transgenic plants. A majority of plants transformed with DN-rop3 under a strong embryonic promoter RPS5A (Weijers et al., 2001) (81% of individual lines [38/47]) displayed embryo phenotype. Analysis of transcription in these DN-rop3 lines by qRT-PCR and immunoblot detection by ROP3 antibody showed that their embryo defects increased with increasing expression of the DN-rop3 transgene (Supplemental Figures 2A to 2D).
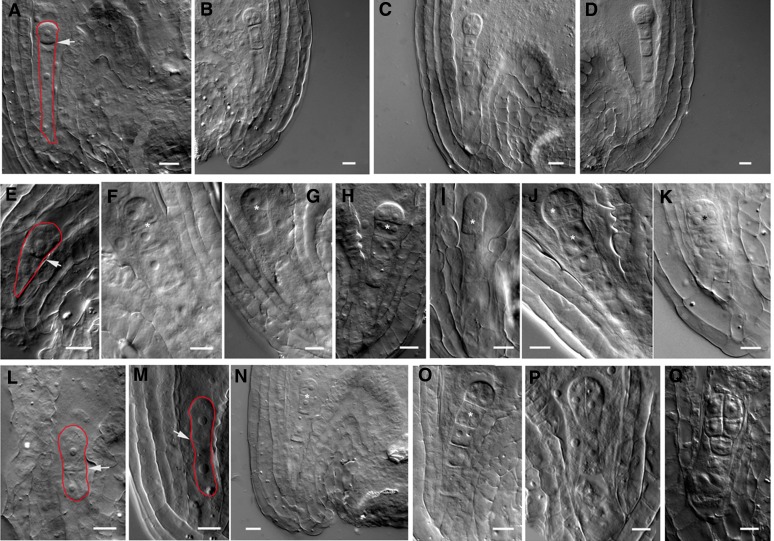
In the embryos, overexpression of DN-rop3 produced a range of cell division defects from one-cell to heart embryo stages (Supplemental Table 2). The wild-type zygote elongates after fertilization and undergoes an asymmetric cell division to result in a small apical cell and a large basal cell (Figure 2A). In contrast to the asymmetric cell division in the wild-type embryos, DN-rop3 zygotes divided more symmetrically, giving rise to almost equal-sized apical and basal cells (Figure 2E). At the two-cell to octant embryo stages, abnormal transverse cell divisions were observed in the apical cell, forming a file of cells (Figures 2F and 2G), while the apical cell in the wild-type embryo divides vertically (Figure 2B) with the basal cell dividing anticlinally to form the suspensor (Figure 2B). At the four-cell stage, suspensor cells displayed aberrant periclinal cell divisions in DN-rop3 embryos (Figures 2H and 2I compared with 2C). When DN-rop3 embryos reached the eight-cell stage, both the apical domain and the suspensor showed cell division defects (Figures 2J and 2K compared with 2D). At 16- and 32-cell stages, tangential divisions separate the protoderm from the inner cells in wild-type embryos (Figures 3A and 3B), whereas DN-rop3 embryos displayed abnormal cell divisions at the apical domain (Figures 3F and 3G). In addition to the phenotypes in the embryo proper, periclinal divisions in the hypophyseal cell and adjacent suspensor cell occurred in DN-rop3 embryos, while no cell division occurred in the hypophysis and normal anticlinal divisions occurred in wild-type suspensors (Figures 3F and 3G compared with 3A and 3B). From the globular to the heart embryo stage, cell division defects in the hypophysis were frequently observed in DN-rop3 embryos, resulting in aberrant organization of the root stem cell niche (Figures 3H to 3J compared with 3C to 3E). Together, the defects in DN-rop3 are consistent with ROP3 being important in promoting the normal pattern formation during embryo development.
Figure 2.
DN-rop3 and rop3 Mutants Display Aberrant Division Patterns in Early Embryogenesis.
(A) to (D) Wild-type embryos at zygotic division (A), two-cell (B), four-cell (C), and eight-cell (D) stages.
(E) to (K) Embryos from RPS5Apro:DN-rop3 transgenic plants at zygotic division (E), two-cell ([F] and [G]), four-cell ([H] and [I]), and eight-cell ([J] and [K]) stages.
(L) to (Q) rop3 embryos at zygotic division ([L] and [M]), two-cell (N), four-cell ([O] and [P]), and eight-cell (Q) stages.
Arrows mark the cell plates between the apical and basal daughter cells of the zygote. Asterisks denote aberrant cell divisions. Red lines outline the zygotes. Bars = 10 μm.
Figure 3.
DN-rop3 and rop3 Mutants Have Defective Cell Divisions in the Basal Lineage of the Embryos.
(A) to (E) Wild-type embryos at 16-cell (A), 32-cell (B), globular (C), triangle (D), and heart (E) stages.
(F) to (J) Embryos of RPS5Apro:DN-rop3 transgenic plants at 16-cell (F), 32-cell (G), globular (H), triangle (I), and heart (J) stages. Arrows indicate the position of aberrant cell division plate.
(K) to (O) rop3 embryos at 16-cell (K), 32-cell (L), globular (M), triangle (N), and heart (O) stages. Bracketed area displays cell division defects in basal embryo region, and the lower one in (G), (K), and (M) shows abnormal cell divisions in suspensor cells.
Bars = 20 μm in (E), (J), and (O) and 10 μm for the rest of the images.
To ascertain the role of ROP3 in embryo development, we obtained lines with T-DNA insertions in the fifth and third exon (SALK_008896 and SALK_0325580C, respectively) of ROP3. These mutants are designated rop3-1 and rop3-2, respectively (Supplemental Figure 3A). qRT-PCR analysis showed absence of ROP3 transcripts in these T-DNA insertion lines, confirming that they are null mutants (Supplemental Figure 3B). Disruption of ROP3 causes defects in asymmetric division of the zygotes (Figures 2L and 2M) and cell division orientation in the apical (Figure 2N) and suspensor cells (Figures 2O to 2Q) during early stages of embryogenesis. rop3 embryos have enlarged suspensors with periclinal cell divisions (Figures 3K and 3M) and abnormal cell divisions in the hypophysis, leading to aberrations in the basal embryo region (Figure 3K to 3O). The embryo phenotype in rop3 knockout mutants was indistinguishable from the phenotype of DN-rop3 embryos, although the severity differed between these mutants. For instance, a higher percentage of zygotes divided symmetrically in rop3 mutants than in DN-rop3 (rop3-1, 16.0%, n = 50; rop3-2, 13.0%, n = 46 versus DN-rop3 L12, 11.7%, n = 60; L16, 12.5%, n = 40; Supplemental Table 2). When these mutant embryos reached later development stages, the percentage of cell division defects in rop3 become lower than DN-rop3 (rop3-1, 8.1%, n = 867; rop3-2, 7.96%, n = 1043 versus DN-rop3 L16, 23.3%, n = 748; L12, 16.01%, n = 537; Supplemental Table 2). The overall lower frequency of cell division defects (Supplemental Table 2) in developing rop3 embryos suggests that other ROPs might be expressed after the zygotic division stage, compensating for the requirement for ROP3.
To verify whether the rop3 embryo phenotype is caused by rop3 deficiency, transgenic plants containing the coding region of ROP3 driven by its endogenous ROP3 promoter (ROP3pro:ROP3) were crossed with rop3-1 and rop3-2 mutants. Homozygous lines were obtained and used for subsequent analysis. The recovered expression levels of ROP3 in rop3-1 and rop3-2 lines were confirmed by qRT-PCR (Supplemental Figure 4A). The ROP3pro:ROP3 transgene can fully rescue the phenotypes of rop3 mutants, including their embryo phenotype (Supplemental Figures 4F to 4K compared with 4C to 4E and Supplemental Table 4 online).
ROP3 Regulates Auxin-Related Postembryonic Development
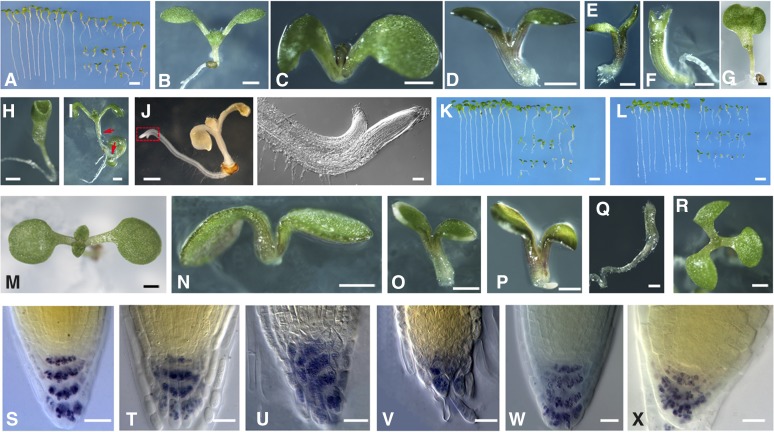
To further investigate the requirement of ROP3 for plant development, we analyzed the seedling phenotype of plants transformed by 35S promoter-driven DN-rop3 (35Spro:DN-rop3) and rop3 mutants. Twenty-five individual 35Spro:DN-rop3 transgenic plants were generated; these plants showed different levels of transgene expression (Supplemental Figures 2B and 2D). Two homozygous lines (L2 and L3), containing a T-DNA insertion at a single locus, were chosen for subsequent analysis. In contrast to the wild type (Figure 4B), progeny from L2 and L3 displayed a variety of developmental aberrations, ranging from altered cotyledon numbers (Figures 4F to 4H) to lack of roots (Figures 4D and 4E) or of both hypocotyls and roots (Figure 4C). Lugol staining of starch granules as a marker for columella cell differentiation revealed a loss of columella stem cells and disorganization of columella cells (Figures 4T to 4V compared with 4S). The most commonly observed seedling phenotype was the development of short roots (Figure 4A, right; Supplemental Table 3), consistent with their having resulted from the disruption of proper patterning in the root stem cell niche (Figures 4T to 4V, compared with 4S). Occasionally, the strong DN-rop3 lines produce offspring with two shoots sharing one primary root (Figure 4I). In some cases, DN-rop3 seedlings have one shoot with two roots fused to each other (Figure 4J). In addition to these root phenotypes, a low percentage of 35S pro:DN-rop3 seedlings (L2, 5.6%, n = 215; L3, 3.4%, n = 232; wild type, 0%, n = 327; Supplemental Table 3) showed severe defects in cotyledon development, including no or single cotyledons, and completely fused cotyledons (Figures 4F to 4H), phenotypes that were also observed in transgenic tobacco with RAC1 downregulated by RNA interference (RNAi) (Tao et al., 2002). The pronounced patterning defects in DN-rop3 seedlings also resemble those of auxin mutants defective in auxin transport and signaling such as pin multiple mutants, gnom, bodenlos, and monopteros (mp) (Friml et al., 2003; Lau et al., 2012).
Figure 4.
DN-rop3 and rop3 Seedling Phenotypes.
(A) Comparison of 7-d-old seedlings between the wild type (left) and progenies of DN-rop3 transgenic plants (right).
(B) Seven-day-old wild-type seedlings.
(C) to (I) Phenotypes of 7-d-old progeny seedlings of DN-rop3 transgenic plants. Red arrows in (I) point to the shoots.
(J) Seven-day-old seedling of DN-rop3 (left), boxed area was magnified (right).
(K) Comparison of 7-d-old seedlings between the wild type (left) and rop3-1 (right).
(L) Comparison of 7-d-old seedlings between the wild type (left) and rop3-2 (right).
(M) Seven-day-old wild-type seedlings.
(N) to (R) rop3 seedlings.
(S) to (X) Lugol-stained root tip of 7-d-old wild type ([S] and [W]), DN- rop3 ([U] to [V]), and rop3 (X) seedlings.
Bars = 1 mm in (B) to (J) and (M) to (R), 5 mm in (A), (K), and (L), 20 μm in (S) to (X).
We also analyzed the seedling phenotype of rop3 mutants. Both rop3-1 (Figure 4K) and rop3-2 (Figure 4L) displayed defects in root growth (Figures 4N to 4P compared with 4M; Supplemental Table 3) and cotyledon development (Figures 4Q and 4R) similar to phenotypes of DN-rop3 transgenic plants. The frequency of defective rop3 seedlings was nevertheless lower than that observed in the progeny of DN-rop3 lines (DN-rop3-L2, 36.3%, n = 215; L3, 41.31%, n = 232; rop3-1, 15.8%, n = 298; rop3-2, 10.64%, n = 356; Supplemental Table 3).
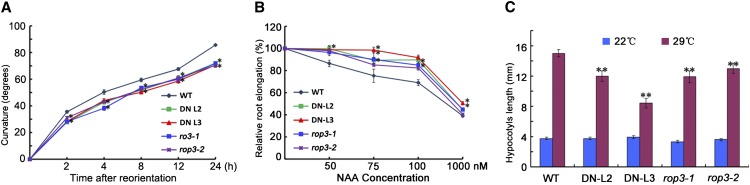
Under normal growth conditions, the progeny of DN-rop3 and rop3 mutants segregated a variety of phenotypes. For instance, some rop3 seedlings displayed severe defects in organ formation, including cotyledons and roots (Figure 4), while some appeared to be normal. We therefore decided to test whether the 35Spro:DN-rop3 and rop3 seedlings, including normal-looking ones, showed altered growth responses to gravity and exogenous auxin. We first examined the root gravitropic responses in DN-rop3 and rop3 mutants. Unlike the wild type, the roots of DN-rop3 and rop3 were less sensitive to gravity, based on measurement of root curvature after gravity stimulation (Figure 5A). We next tested whether the sensitivity to applied auxin was also affected in DN-rop3 and rop3. Exogenous auxin inhibited root elongation of wild-type seedlings, but DN-rop3 and rop3 roots were more resistant to auxin inhibition over a range of hormone concentrations (Figure 5B). The difference from the wild type was especially notable at low auxin concentrations, e.g., 50 nM NAA, where the root length of DN-rop3 and rop3 was almost not affected compared with 15% growth inhibition shown by wild-type seedlings (Figure 5B). At 100 nM NAA, wild-type root length was reduced to 69% of that of untreated seedlings, whereas DN-rop3 and rop3 roots displayed considerably less inhibition, with root length being around 90% and over 80%, respectively, of that of the corresponding untreated seedlings (Figure 5B), indicating that DN-rop3 and rop3 mutants have impaired auxin responses.
Figure 5.
DN-rop3 and rop3 Mutants Display Reduced Responses to Auxin.
(A) Time course of curvature in root gravitropic response tests. Root gravitropic responses of 35Spro:DN-rop3 transgenic lines (L2 and L3) and rop3 knockout mutants (rop3-1 and rop3-2) are compared with the wild-type controls. Curvatures were measured at different time as indicated after reorientation. Data are means ± sd (n = 30 to 50). Asterisks indicate significant differences from the wild type by Student’s t test (*P < 0.05; **P < 0.01).
(B) Measurement of relative root elongation of seedlings grown on medium supplemented with different concentrations of NAA compared the sensitivity to auxin of 35Spro:DN-rop3 transgenic lines (L2 and L3), rop3 knockout mutants (rop3-1 and rop3-2), and wild-type control. Primary root lengths of untreated plants were set as 100%. Data are means ± sd (n = 30 to 50). Asterisks indicate significant differences from the wild type by Student ’s t test (*P < 0.05; **P < 0.01).
(C) Hypocotyl lengths of 7-d-old seedlings grown at 22 and 29°C were measured for 35Spro:DN-rop3 transgenic lines (L2 and L3), rop3 knockout mutants (rop3-1 and rop3-2), and wild-type control. Data are means ± sd (n = 30 to 50). Asterisks indicate significant differences from the wild type by Student's t test (*P < 0.05; **P < 0.01).
We also analyzed the effect of hypocotyl elongation under high temperature (29°C), another well-established auxin-dependent process because high temperature has been shown to induce higher level of auxin and therefore stimulates hypocotyl elongation (Gray et al., 1998). Seedlings of DN-rop3 and rop3 mutants were clearly less responsive to high temperature in stimulated hypocotyl elongation than the wild type (Figure 5C), suggesting repressed auxin responses in DN-rop3 and rop3 mutants.
As ROP3 is expressed in pollen (Supplemental Figure 1C) and therefore likely plays a role in pollen development similar to the closely related genes ROP1 and ROP5 (Craddock et al., 2012). Our analysis of rop3 mutants did not show any notable pollen development phenotype, germination in vivo, and tube growth properties in the pistil (Supplemental Figure 5). However, we could not rule out the possibility that ROP3 functions in pollen, since two other pollen-expressed ROPs, ROP1 and ROP5, are abundantly expressed there. In fact, ROP1 and ROP5 had been shown to play roles in pollen tube polar growth by gain-of-function studies (Kost et al., 1999; Li et al., 1999; Gu et al., 2003)
ROP3 Regulates the Establishment of Auxin Maxima in the Embryos and Roots
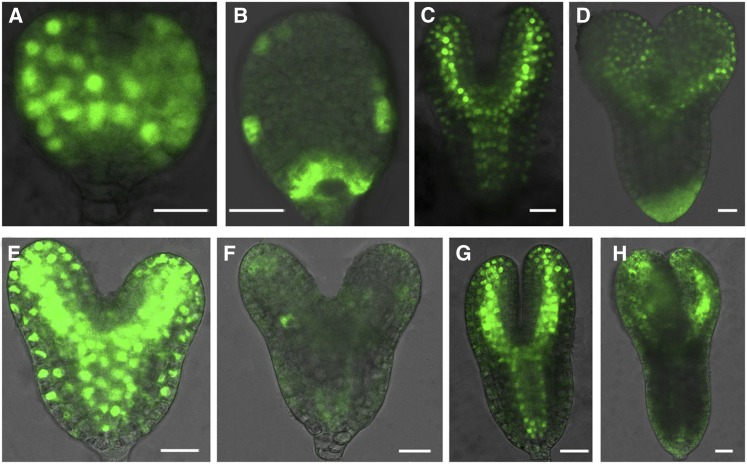
Phenotypic analyses (Figures 2 to 5) suggest that auxin distribution and responses are affected in DN- rop3 and rop3 mutants. To explore this, we analyzed the expression of the auxin reporter DR5rev:GFP (for green fluorescent protein; Benková et al., 2003) in the DN-rop3 and rop3 backgrounds. As expected, an auxin maximum was detected at the root pole of wild-type embryos (100%, n = 53; Figure 6A). In contrast, this auxin maximum was strongly reduced in a large majority of DN-rop3 embryos (63/66; Figure 6B), although in a small number (3/66) of embryos, the DR5rev:GFP signal was spread out everywhere (Figure 6C). In rop3 embryos, we also observed reduced DR5 activity at the root pole (49/52; Figure 6E) and altered pattern of DR5 activity in a small number (3/52) of the analyzed embryos (Figure 6F).
Figure 6.
ROP3 Regulates the Maintenance of the Auxin Maximum.
(A) to (C) DR5rev:GFP expression in heart embryos of the wild type (A) and RPS5Apro:DN-rop3 ([B] and [C]).
(D) to (F) DR5rev:GFP expression in embryos of the wild type (D) and rop3 ([E] and [F]).
(G) to (J) DR5rev:GFP expression in roots of 4-d-old wild-type ([G] and [I]), 35Spro:DN-rop3 (H), and rop3 (J) seedlings.
Bars = 20 μm in (A) to (F) and 50 μm in (G) to (J).
We next examined DR5 activity in DN-rop3 and rop3 mutant roots. In comparison to wild type, the DR5 activity was highly suppressed in DN-rop3 (Figure 6H compared with 6G) and was clearly albeit less drastically reduced in rop3 roots (Figure 6J compared with 6I). These observations suggested that the establishment of auxin maxima was disturbed in DN-rop3 and rop3 embryos and roots, correlating with aberrant cell divisions observed in the basal region of their embryos and root tips.
ROP3 Regulates the Expression of MP and PLT1/ PLT2
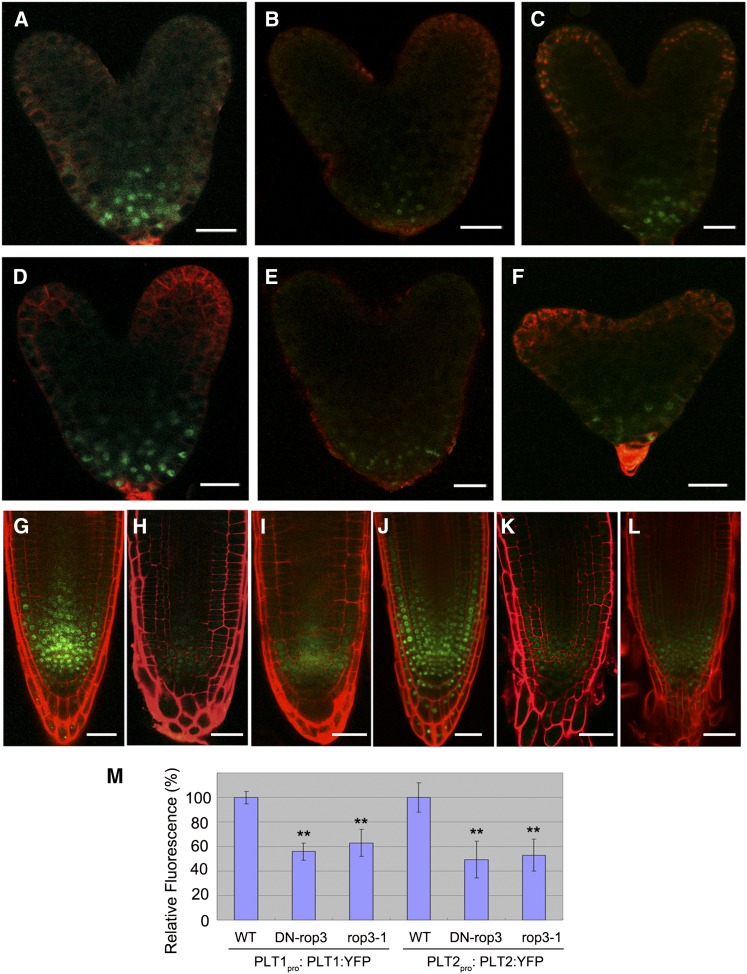
The phenotypes of DN-rop3 and rop3 mutants, together with DR5rev:GFP expression defects, suggest that ROP3 participates in auxin-mediated embryo patterning and root growth. To further address this issue, we focused on whether ROP3 affects some of the regulators that control cell fate specification events in embryogenesis and root meristem establishment, including MP, encoding auxin response factor 5, and root stem cell determinants PLT1 and PLT2, whose expression is dependent on auxin (Aida et al., 2004; Galinha et al., 2007). We first analyzed MP expression, as revealed by the marker MPpro:n3XGFP (Rademacher et al., 2012), in DN-rop3 and rop3 mutant embryos. The expression of MP was drastically altered in both DN-rop3 (Figures 7B and 7D compared with 7A and 7C) and rop3 embryos (Figures 7F and 7H compared with 7E and 7G). Next, we investigated whether ROP3 was involved in the regulation of PLTs and examined the expression of PLT1 and PLT2 in DN-rop3 and rop3 embryos. The expression of PLT1pro:PLT:YFP and PLT2 pro:PLT2:YFP was significantly reduced in DN-rop3 and rop3 embryos from the levels seen in the wild type (Figures 8A to 8F and 8M); these data indicate that ROP3 is important for maintaining MP and PLT1/PLT2 expression and imply a role for this GTPase in the regulation of cell fate identity and embryo polar axis formation.
Figure 7.
ROP3 Affects Embryonic Expression of MP.
(A) to (D) MPpro:3XGFP is expressed in the wild type ([A] and [C]) and RPS5Apro:DN-rop3 ([B] and [D]) at triangle and torpedo stages, respectively.
(E) to (H) MPpro:3XGFP is expressed in the wild type ([E] and [G]) and rop3 ([F] and [H]) at heart and torpedo stages, respectively.
Bars = 20 μm.
[See online article for color version of this figure.]
Figure 8.
ROP3 Mutations Affect the Expression of PLT1 and PLT2.
(A) to (C) The expression of PLT1 pro:PLT1:YFP in embryos of the wild type (A), RPS5Apro:DN- rop3 (B), and rop3 (C).
(D) to (F) The expression of PLT2 pro:PLT2:YFP in embryos of the wild type (D), RPS5Apro:DN-rop3 (E), and rop3(F).
(G) to (I) The expression of PLT1 pro:PLT1:YFP in roots of the wild type (G), 35Spro:DN-rop3 (H), and rop3 (I).
(J) to (L) The expression of PLT2 pro:PLT2:YFP in roots of the wild type (J), 35Spro:DN-rop3 (K), and rop3 (L).
(M) Quantification of PLT1pro:PLT1:YFP and PLT2pro:PLT2:YFP fluorescence intensity as shown in (A) to (F). Data shown are average and sd (n = 20 to 30). Asterisks indicate Student's t test significant difference between the wild type and mutant embryos (**P < 0.01).
Bars = 20 μm.
Downregulation of PLT1:YFP was observed in 62.5% of DN-rop3 (n = 40) and 27.8% of rop3 roots (n = 36) compared with the wild type (0%, n = 30) (Figures 8G to 8I). Similarly, the expression of PLT2: YFP was altered in 40.0% of DN-rop3 (n = 30) and 20.8% of rop3 (n = 48) in contrast to the wild type (0%, n = 30) (Figures 8J to 8L), indicating that the observed seedling root meristem defects in rop3 mutants had resulted from the altered PLT1 and PLT2 expression.
ROP3 Regulates the Polar Localization and Expression of PIN1 and PIN3
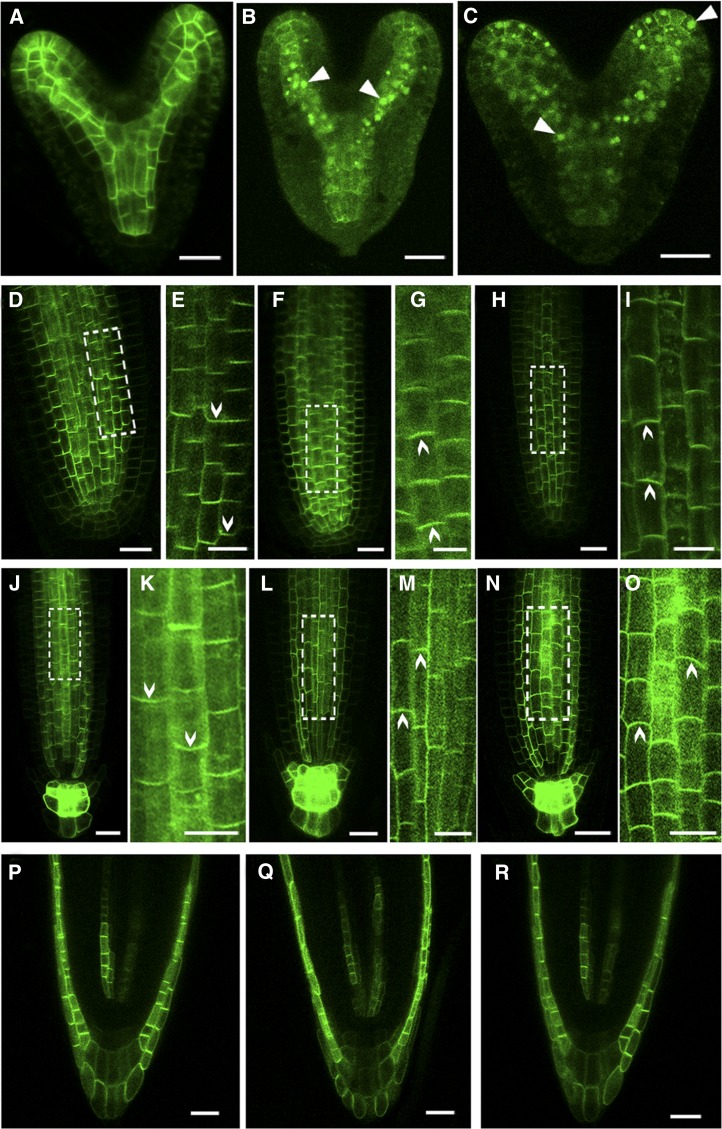
We next determined whether the altered DR5 activity during embryogenesis and root growth in DN-rop3 and rop3 mutants associate with alterations in auxin transport. The expression and localization of PIN1pro:PIN1:GFP was examined in DN-rop3 and rop3 embryos. Wild-type embryos showed the normal accumulation of PIN1:GFP (Figure 9A), while a severe reduction of PIN1:GFP in the PM was observed in the cotyledons and provascular cells of the central axis in DN-rop3 and rop3 mutant embryos (Figures 9B and 9C). Reduced presence of PIN1:GFP from the PM apparently led to the loss of polarity of PIN1:GFP distribution in the affected cells (Figures 9B and 9C). Moreover, PIN1:GFP labeling was found within intracellular aggregates in the embryo cells in DN-rop3 and rop3 mutants (Figures 9B and 9C), indicating that ROP3 is required for the localization of PIN1 to the PM, thereby affecting polar auxin transport during embryo development.
Figure 9.
ROP3 Is Required for the Polar Localization of PINs.
(A) to (C) PIN1pro:PIN1:GFP localization in embryos of the wild type (A), RPS5Apro:DN-rop3 (B), and rop3 (C). Arrowheads indicate intracellular aggregates of PIN1 protein.
(D) to (I) PIN1pro:PIN1:GFP localization in roots of 4-d-old wild-type ([D] and [E]), 35Spro:DN-rop3 ([F] and [G]), and rop3 ([H] and [I]) seedling. Regions in white boxes (D), (F), and (H) are confocal scans with high magnifications shown in (E), (G), and (I). Arrowheads indicate the direction of PIN1 polarity.
(J) to (O) PIN3 pro:PIN3:GFP localization in roots of 4-d-old wild-type ([J] and [K]), 35Spro:DN-rop3 ([L] and [M]), and rop3 ([N] and [O]) seedling. Regions in white boxes ([K], [M], and [O]) are confocal scans at high magnification. Arrowheads indicate the direction of PIN3 polarity.
(P) to (R) AUX1pro:AUX1:YFP localization in roots of 4-d-old wild-type (P), 35Spro:DN-rop3 (Q), and rop3 (R) seedling.
Bars = 20 μm.
[See online article for color version of this figure.]
We also analyzed the localization and expression of PIN1:GFP, PIN2:GFP, and PIN3:GFP in the roots of DN-rop3 and rop3 mutants. In wild-type roots, PIN1:GFP was localized in the basal membrane of the stele cells (100%, n = 34; Figures 9D and 9E). However, the polarity of PIN1:GFP was changed in the root stele cells of DN-rop3 (41.3%, n = 80) and rop3 (20%, n = 60) mutants, where PIN1:GFP was mainly detected in the apical PM instead of the basal side of the stele cells (Figures 9F to 9I). In addition, there were ∼30% (n = 80) of DN-rop3 and 15% (n = 60) of rop3 mutant roots, which displayed reduced PIN1:GFP accumulation (Supplemental Figures 6A to 6C). Similarly, we analyzed the expression and localization of PIN3:GFP in the roots of the DN-rop3 and rop3 mutants. Unlike in wild-type roots, which all showed normal accumulation of PIN3pro:PIN3:GFP (100%, n = 37; Figures 9J and 9K), DN-rop3 (28%, n = 50) and rop3 roots (24%, n = 32) showed a basal to apical shift of PIN3 localization in root stele cells (Figures 9L to 9O). DN-rop3 (24%, n = 50) and rop3 roots (22%, n = 32) also showed reduced abundance of PIN3:GFP in the stele and root columella cells (Supplemental Figures 6E and 6F) relative to the wild type (Supplemental Figure 6D). Our previous work on the ROP activator RopGEF7 showed that downregulation of RopGEF7 by the RNAi technique resulted in reduced accumulation of PIN1pro:PIN1:GFP in embryos and roots (Chen et al., 2011). Here, we also examined the polarity of PIN1 and PIN3 in these RopGEF7RNAi roots. However, the polarity of PIN1pro:PIN1:GFP in RopGEF7RNAi roots was not obviously different from that in the wild type (Supplemental Figures 9A and 9B). Similarly, we observed reduced PIN3pro:PIN3:GFP accumulation, but no obvious polarity change in RopGEFRNAi roots compared with the wild type (Supplemental Figures 9C and 9D).
To determine whether the reduced PIN1 and PIN3 protein abundance in DN-rop3 and rop3 mutant roots is due to altered PIN transcript level, we examined the RNA transcript of the PIN1 and PIN3 genes in wild-type, DN-rop3, and rop3 roots. qRT-PCR analysis showed that the expression levels of PIN1 and PIN3 in DN-rop3 and rop3 mutant roots were not substantially different from the wild type (Supplemental Figure 7), suggesting that downregulation of PIN1 and PIN3 proteins in DN-rop3 and rop3 mutant roots occur at the posttranscriptional level.
Two other auxin transporters are not affected by ROP3 mutations. PIN2:GFP, which localizes to the basal side of the root cortical cells and the apical side of the epidermis cells in the wild-type roots (Supplemental Figure 8A) did not change its polarity in DN-rop3 and rop3 mutant roots (Supplemental Figures 8B and 8C). The localization of auxin influx carrier AUX1-YFP, which is apically localized in the wild-type root protophloem cells (Figure 9P), is also not altered in DN-rop3 and rop3 mutants (Figures 9Q and 9R compared with 9P). We also confirmed that ROP3 mutations had no effect on the transcript levels of PIN2 and AUX1 genes compared with the wild type (Supplemental Figure 7). These observations demonstrate that ROP3 specifically regulates the level and localization of PIN1 and PIN3 in the roots. Taken together, our data suggest that ROP3 regulates auxin distribution in embryos and seedling roots and that some DN-rop3 and rop3 phenotypes could have resulted from changes in PIN1 and PIN3 polarity or level.
ROP3 Regulates the Recycling of PIN1 and PIN3 to the PM
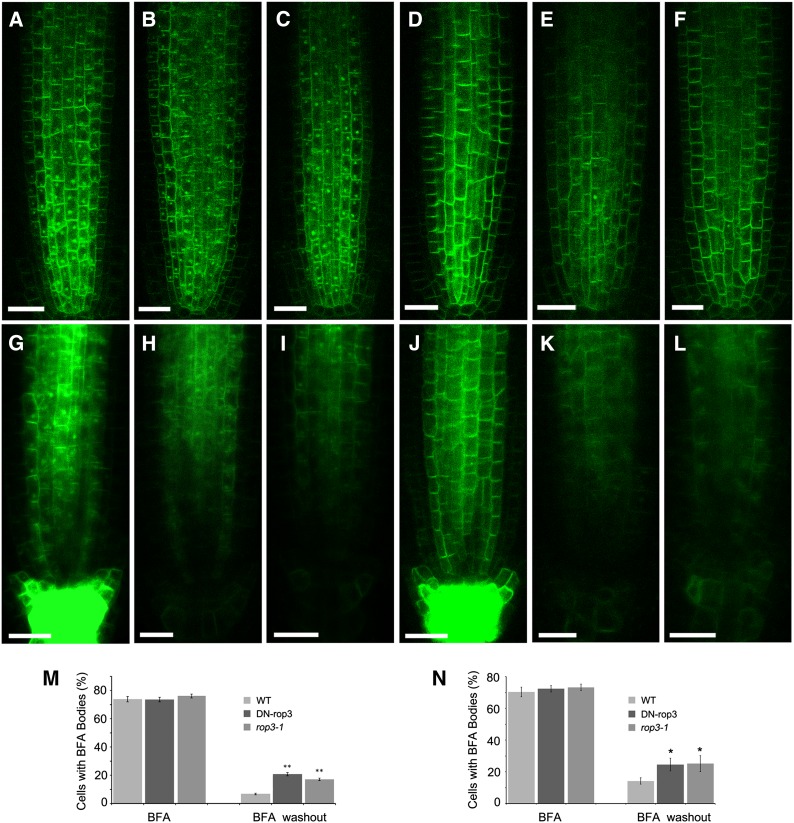
PIN proteins continuously cycle between endosomes and the PM (Steinmann et al., 1999; Geldner et al., 2003). The reduced enrichment of PIN1 and PIN3 in the PM in DN-rop3 and rop3 mutants (Figure 9; Supplemental Figure 6) suggests that ROP3 is involved in the recruitment of PINs to the PM. To address this possibility, PIN1:GFP trafficking was investigated using brefeldin A (BFA), a vesicle trafficking inhibitor that inhibits PIN recycling and results in the formation of membrane protein aggregates referred to as BFA compartments (Geldner et al., 2001). BFA compartment formation was examined in the wild-type, DN-rop3, and rop3 mutants. After BFA treatment (100 μM) for 1 h, similar levels (between 73.5 and 76.1%) of seedling root cells accumulated PIN1:GFP-containing BFA compartments in wild-type, DN-rop3, and rop3 mutants (Figures 10A to 10C and 10M). The effects of BFA treatment on vesicle trafficking are reversible. After washout of BFA for 90 min, BFA compartments were still detected in 20.7 and 17.1% of root cells in DN-rop3 and rop3 mutants (Figures 10E and 10F), respectively, compared with in only 6.7% of wild-type root cells (Figures 10D and 10M). We next analyzed whether ROP3 mutations affected the trafficking of PIN3:GFP and observed a similar result to PIN1:GFP for PIN3:GFP at BFA treatment (Figures 10G to 10I and 10N) and washout conditions (Figures 10J to 10L and 10N). However, PIN2:GFP recycling was not affected in wild-type, DN-rop3, or rop3 roots (Supplemental Figures 10A to 10G). Additionally, the trafficking of a PM marker, water channel protein PIP2:GFP, was not impaired in DN-rop3 and rop3 mutants in contrast to the wild type (Supplemental Figures 11A to 11G), suggesting that ROP3 regulates recycling of specific PIN proteins but does not affect the trafficking of PIN2 and general PM proteins. Thus, it is likely that DN-rop3 and rop3 phenotypes are associated with alteration of auxin transport and PIN1 and PIN3 function.
Figure 10.
ROP3 Affects the Trafficking of PIN1:GFP and PIN3:GFP in Roots.
(A) to (C) BFA-treated seedlings expressing PIN1-GFP at 3 d after germination of the wild type (A), 35Spro:DN-rop3 (B), and rop3 (C).
(D) to (F) BFA washout in PIN1:GFP (D), DN-rop3 (E), and rop3 (F).
(G) to (I) BFA-treated seedlings expressing PIN3:GFP at 3 d after germination of the wild type (G), 35Spro:DN-rop3 (H), and rop3 (I).
(J) to (L) BFA washout in PIN3:GFP (J), 35Spro:DN-rop3 (K), and rop3 (L).
(M) and (N) Percentage of stele cells with PIN1:GFP-labeled (M) or PIN3:GFP-labeled (N) BFA bodies before and after BFA washout in the wild type, 35Spro:DN-rop3, and rop3. Data are means ± sd (n = cell numbers from 15 to 30 roots), and asterisks indicate significant differences by Student’s t test (**P < 0.01; *P < 0.05).
Bars = 20 μm.
[See online article for color version of this figure.]
Inhibition of Proteasome Activity Enhances PIN1:GFP and PIN3:GFP Accumulation in DN-rop3 and rop3 Mutants
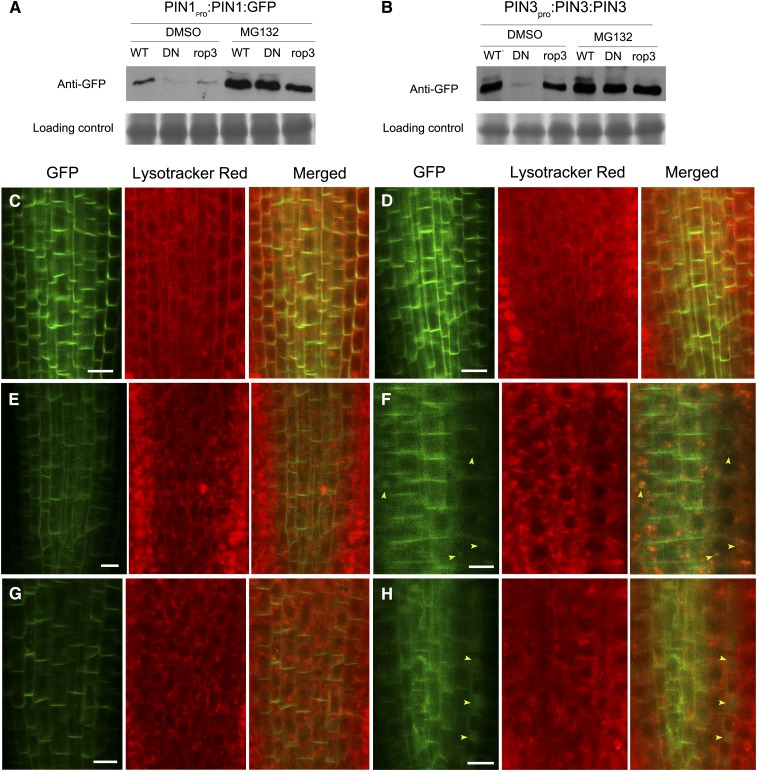
As ROP3 mutations do not interfere with the transcription of PIN1 and PIN3 (Supplemental Figure 7) but affect the recruitment of PIN1 and PIN3 to the PM (Supplemental Figures 6 and 10), it is likely that the reduced GFP-tagged PIN1 and PIN3 signals in rop3 mutants are associated with protein degradation. We examined the PIN1:GFP and PIN3:GFP protein levels in rop3 mutants by immunoblotting analysis and detected small, comparable amounts of PIN1:GFP in DN-rop3 and rop3 lines, in contrast to the larger amounts detected in the wild type (Figures 11A and 11B). The PIN3:GFP level was affected more in DN-rop3 mutants than in the rop3 line. This observation might reflect that PIN1 accumulation is largely dependent on ROP3, whereas PIN3 accumulation is affected by ROP3 and also most probably by other ROPs. Figures 11A and 11B also showed that MG132-treated seedlings had an increase at PIN1:GFP and PIN3:GFP protein levels in wild-type, DN-rop3, and rop3 compared with untreated controls. Since previous studies showed PIN proteins were targeted to vacuoles for degradation (Kleine-Vehn et al., 2008a), we therefore also examined the cellular fate of these PINs in DN-rop3 and rop3 seedlings using the fluorescent dye lysotracker red, which specifically marks acidic endomembrane compartments (Laxmi et al., 2008). In these MG132-treated seedling roots, we observed PIN1:GFP signal in circle-like structures that overlapped with the vacuoles labeled with lysotracker red in DN-rop3 (22.2%, n = 36; Figure 11F) and rop3 (16.2%, n = 37; Figure 11H) compared with none in the wild type (0%, n = 30; Figure 11D). The DMSO-treated control wild-type, DN-rop3, and rop3 roots did not show vacuole-localized signal (Figures 11C, 11E, and 11G). These data suggest that changes in PIN1:GFP protein abundance in rop3 mutants might associate with protein degradation that partially occur at the vacuoles. On the other hand, PIN3:GFP did not accumulate notably in the vacuoles of MG132-treated rop3 mutants (Supplemental Figure 12).
Figure 11.
PIN1:GFP Partially Accumulates in Vacuoles in MG132-Treated Roots of 35Spro:DN-rop3 and rop3.
(A) and (B) PIN1: GFP (A) and PIN3:GFP (B) protein levels in MG132-treated and untreated wild-type, DN-rop3, and rop3 seedlings. DN represents 35Spro:DN-rop3. Immunoblot was performed using anti-GFP antibody. Lower panels indicate Coomassie blue staining of the protein samples as loading controls
(C) and (D) PIN1:GFP localization in roots of 5-d-old seedlings not treated (C) or treated with MG132 (D).
(E) and (F) PIN1:GFP localization in roots of 35Spro:DN-rop3 seedlings not treated (C) or treated with MG132 (D).
(G) and (H) PIN1:GFP localization in roots of rop3 seedlings untreated (E) or treated with MG132 (F).
Lysotracker red was used for labeling of vacuolar compartments. Arrowheads mark the vacuoles containing PIN1:GFP. Bars = 10 μm.
DISCUSSION
ROP3 Regulates Auxin-Dependent Plant Development
ROP GTPases have been implicated in auxin signaling (Wu et al., 2011). However, details on the contributions of specific ROPs to auxin-regulated development has been sparse. The results presented here demonstrate that ROP3 plays a key role in auxin-regulated development throughout embryogenesis (Figures 2 and 3) and in postembryonic processes (Figures 4 and 5), including the gravitropic response (Figure 5A), auxin-suppressed root growth (Figure 5B), and sensitivity to auxin-regulated hypocotyl elongation at elevated temperature (Figure 5C). In showing that ROP3 is important for maintaining the DR5rev:GFP-marked auxin-responsive maxima (Figures 6A to 6F), the expression of MP/AUXIN RESPONSE FACTOR5 (ARF5) (Figures 7A to 7H) and PLT1/2 (Figures 8A to 8M), our results provide insights on the cellular and physiological processes that underpin the developmental role of ROP3.
Taken together with the developmental phenotypes observed in DN-rop3 and rop3 mutants, the expression pattern of ROP3 is consistent with this small GTPase being important in regulating auxin-mediated patterning during embryogenesis (Figures 2 and 3), organ formation, and seedling growth (Figures 4 and 5). The dynamic expression pattern of ROP3pro:GUS, shifting from an apical to a basal high from early to developed embryos (Figure 1), strongly resembles changes in auxin distribution, as reflected by DR5rev:GFP accumulation during embryogenesis (Friml et al., 2003). ROP3 peak activity in the embryo and root (Figure 1) corresponds to the sites of auxin maxima in the hypophysis, quiescent center/columella initials, and cotyledon primordia, consistent with an important role for ROP3 in embryo development. Moreover, postembryonically, ROP3 expression (Figure 1; Supplemental Figure 1) overlapped with those of PIN1 and PIN3 in the root (Blilou et al., 2005; Vieten et al., 2005) and appeared similar to PIN3 in root columella cells (Blilou et al., 2005; Vieten et al., 2005). Furthermore, the failure of DN-rop3 to recruit PIN1 and PIN3 proteins to the PM in roots (Supplemental Figure 6) occurred along with diminished cell membrane localization, as suggested by the cytoplasmic and perinuclear localization of YFP:DN-rop3 (Supplemental Figures 1G and 1H). This observation is consistent with cell membrane association of ROP3 (Supplemental Figures 1E and 1F) being important for the recruitment of PINs to the PM. Taken together with PINs being the predominant auxin transporter in the stele, the ROP3 expression pattern is consistent with its involvement in the regulation of PIN-mediated auxin transport in the root, thereby regulating seedling development. Furthermore, the observation that ROP3 expression in the root is enhanced in response to auxin induction (Figures 1I and 1J) is also consistent with ROP3 engaging in a regulatory loop responsive to auxin, augmenting its importance in auxin-mediated organogenesis and growth.
ROP3 Functions in the Regulation of PIN Polarity and Trafficking
Several recent observations indicated a link between ROP function and PIN endocytosis (Chen et al., 2012; Lin et al., 2012; Nagawa et al., 2012). Our results (Figure 10) show that downregulating ROP signaling or loss of ROP3 function disrupts PIN1 and PIN3 recycling. Together, these results imply that different ROPs could perform distinct functions in the regulation of PIN trafficking, thereby leading to altered PIN polarity and perturbing directional auxin transport. Moreover, our results also show that ROP3 differentially affects auxin transporters, even within the PIN family (Figure 9; Supplemental Figure 8). On the other hand, despite overlapping with AUX1 in its expression domain, ROP3 has no impact on the polarity of AUX1 (Figures 9P to 9R). These data suggest that ROP3 specifically affects the localization of PIN proteins in regions of the root where they coexpress during development.
ROPs are activated by auxin (Tao et al., 2002; Xu et al., 2010) and activated ROPs interact with effectors, such as RIC1 and ICR1, that have been linked to auxin signaling. Among these ROP effectors, evidence convincingly supports that ICR1 regulates the exocytic trafficking of PIN proteins to polar domains in the PM and is required for auxin transport, most strongly impacting embryo and root meristem patterning (Hazak et al., 2010). On the other hand, phenotypes of downregulated RIC1 mutants were relatively mild, e.g., only moderately enhanced lateral root development and weak defects in leaf vasculature were reported for ric1 (Chen et al., 2012; Lin et al., 2012). It is therefore likely that ICR1 could act as a downstream effector of ROP3, mediating auxin-regulated responses. Future studies will be needed to elucidate whether ROP3 connects with the ICR1-dependent downstream pathway resulting in PIN polarization.
Another possible mechanism for how ROP3 might regulate the polarity of PIN proteins via PID. PID and related AGC kinases had been reported to promote transcytosis-directed apical PIN delivery through phosphorylation of PIN proteins (Dhonukshe et al., 2010). The activity of PID is stimulated by phospholipid signaling (Zegzouti et al., 2006). In pollen tubes, ROPs have been demonstrated to be associated with phosphatidylinositol monophosphate kinase (PtdIns p-K) activity (Kost et al., 1999). This might be mediated through their C-terminal polybasic domain, a feature common to RAS-related small GTPases and known to interact with both phosphatidylinositol 4,5-bis phosphate and phosphatidylinositol 3,4,5-triphosphate (Heo et al., 2006). Based on these observations, even if ROP-mediated PIN polarity might not directly target PID, it could provide a potential integration point for multiple cellular processes to influence auxin-dependent development through ROP signaling.
PIN protein abundance in the PM can be controlled by endocytosis or exocytosis (Löfke et al., 2013). Some PINs are targeted to the lytic vacuole in a retromer-dependent manner and degraded (Kleine-Vehn et al., 2008a). Our BFA treatment and washout experiments suggest that ROP3 mutations specifically affect PIN1 and PIN3 recycling back to the PM (Figure 10; Supplemental Figures 10 and 11). The recycling defects of PIN1 and PIN3 in rop3 mutants could apparently trigger protein degradation. Treatment with the 26S proteasome inhibitor MG132 enhances PIN1 protein accumulation at the cytoplasm and in the vacuoles (Figures 11C to 11F) in DN-rop3 and rop3 mutants. However, we could not find vacuolar accumulation of PIN3 in DN-rop3 and rop3 mutants (Supplemental Figure 12). At present, the precise mechanism on how ROP3 recruits PIN proteins is not known; we expect future studies of ROP3 downstream interacting proteins to shed light on its roles in the control of cell polarity of the polar auxin transport system.
How ROP3 activity is regulated so as to impact the auxin-related processes described here remains unknown. AUXIN BINDING PROTEIN1 (ABP1) has been suggested to act upstream of ROP6 and ROP2, which regulate PIN1 internalization and mediate the auxin signal to activate these small GTPases (Xu et al., 2010). ABP1 has also been shown to negatively regulate the auxin-controlled SCFTIR1/AFB pathway (Tromas et al., 2013). It therefore remains to be seen whether ABP1 is part of the ROP3-mediated signaling pathway.
Studies involving receptor-like kinases have provided support for a model whereby RopGEFs serve as linkages between cell surface signal perception and ROP activation (Kaothien et al., 2005; Nibau et al., 2006; Zhang and McCormick, 2007; Zhang et al., 2008; Duan et al., 2010; Nibau and Cheung, 2011). In these studies, two related pollen-specific receptor-like kinases from tomato (Solanum lycopersicum) and Arabidopsis have been shown to interact with RopGEFs, mediating similar ROP-induced phenotypes in pollen tubes. Additionally, the FERONIA receptor kinase (FER) was identified as a regulator of auxin-stimulated NADPH oxidase-dependent reactive oxygen species-mediated root hair tip growth (Duan et al., 2010), an auxin- and ROP-regulated process (Foreman et al., 2003). FER interacts with RopGEFs, and RopGEF7 (Duan et al., 2010) regulates accumulation of PIN1 protein in embryonic and root cells and is required for the formation of auxin maxima (Chen et al., 2011). RopGEF7 is expressed in quiescent center cells and interacts with ROP3 (Chen et al., 2011) and both RopGEF7 and ROP3 are transcriptionally induced by auxin. Our results (Figure 1) show that ROP3 is also expressed in the hypophysis and its daughter cells. Therefore, FER, RopGEF7, and/or other members of these protein families that are expressed in the same tissue domain as ROP3 could act as upstream regulators of the ROP3-regulated processes described here. Future studies will decipher the mechanisms that underlie how the ROP3 switch is regulated.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana plants used in this study were Columbia-0 ecotype. The DR5rev:GFP (Benková et al., 2003), PIN1pro:PIN1:GFP (Benková et al., 2003), PIN2pro:PIN2:GFP (Blilou et al., 2005), PIN3pro:PIN3:GFP (Blilou et al., 2005), AUX1pro:AUX1:YFP (Swarup et al., 2004), 35S1pro: PIP2:GFP (Cutler et al., 2000), PLT1 pro:PLT1:YFP, PLT2pro:PLT2:YFP (Grieneisen et al., 2007), and MPpro:n3xGFP (Rademacher et al., 2012) constructs have been described previously.
Seeds were surface-sterilized and plated on half-strength Murashige and Skoog (MS) medium containing 1.5% sucrose and 0.8% agar. Seeds on one-half MS plates were stratified at 4°C for 2 to 3 d in darkness and transferred to a phytotron set with a 16-h-light/8-h-dark cycle at 22°C. After 7 to 10 d, seedlings were transferred to soil and grown under the same conditions.
Vector Construction and Plant Transformation
ROP3pro:GUS was generated by insertion of a 1.034-kb PCR-amplified ROP3 promoter sequence into pBI101.1 at the SalI and BamHI sites. DN-rop3 mutation with an A121D conversion in ROP3 was created by site-specific mutagenesis of the ROP3 cDNA subcloned in a Bluescript pSK vector. The mutant version of ROP3 (DN-rop3) was then cloned into the pAC1352 binary vector carrying cauliflower mosaic virus 35S promoter and a modified pCambia1300 containing the embryo-specific RPS5A promoter, which have been previously described (Chen et al., 2011).
The above constructs were introduced into Agrobacterium tumefaciens strain C58, which were used for transformation of Arabidopsis by the floral dip method (Clough and Bent, 1998). The sequences of the primers used in the vector construction are listed in Supplemental Table 1.
Identification of rop3 Mutants and Complementation of ROP3
Two Arabidopsis T-DNA insertion lines for rop3-1 (SALK_008896) and rop3-2 (SALK_032558C) were obtained from ABRC. A PCR-based approach was used to confirm the T-DNA insertion and to identify the homozygous lines. Transcript levels in mutants and wild-type plants were analyzed by qRT-PCR analysis. Primers used for the identification of the mutants are listed in Supplemental Table 1.
The ROP3 promoter was subcloned into a modified PBI101 vector to generate ROP3pro:ROP3 harboring cDNA of ROP3. The construct was transformed into wild-type plants and homozygous lines were crossed with rop3 mutants for complementation studies. Transcript levels of ROP3 in rop3 mutants were confirmed by qRT-PCR.
NAA Treatment
Wild-type seeds were surface sterilized and germinated on half-strength MS agar plates. Five- to seven-day-old seedlings were treated with 10 μM NAA for 0, 6, 12, and 24 h, respectively, and roots were harvested for RNA extraction. Expression levels of ROP3 were analyzed by qRT-PCR.
qRT-PCR Analysis
For analysis of ROP3 gene expression, total RNA was extracted from embryos and 7- to 10-day-old seedlings using the RNeasy plant mini kit (Qiagen). For the analysis of PIN1, PIN2, PIN3, and AUX1 transcripts in wild-type, DN-rop3, and rop3 mutant plants, root samples from 7-d-old seedlings were collected and subjected to total RNA extraction. Root longitudinal section preparation and RNA extraction were performed as described by Dinneny et al. (2008) with some modifications. Roots of 5-d-old seedlings were cut into three regions using a scalpel. The first cut was made ∼350 μm from the root tip at the point of the end of the meristematic zone (Zone 1); the second cut was made ∼200 to 300μm above the first cut, below the region where root hairs emerge (Zone 2-elongation zone); the third cut was made ∼1 mm above the second cut (Zone 3-maturation zone). Forty roots were collected per replicate. Two replicates were performed per zone. RNA was extracted using the RNAprep Pure Micro Kit (Tiangen).
cDNA was prepared from 3 μg of total RNA with PrimeScript reverse transcriptase (TakaRa). PCR reaction was performed on an Illumina Eco (Illumina) system with a SYBR probe (TAKARA). Expression of ROP3, PIN1, PIN2, PIN3, and AUX1 in transgenic or mutant plants were normalized to the expression of ACTIN2 and then compared with the wild type. Data presented are the averages from at least three biological replicates with sd.
A list of gene-specific primers used is provided in Supplemental Table 1.
Immunoblot Analysis
Embryos and 7-d-old wild-type seedlings and seedlings containing RPS5Apro:DN-rop3 and 35Spro:DN-rop3 constructs, respectively, were collected for total protein extraction. Anti-ROP3 antibody (Abmart) was used for the detection of ROP3. For PIN1:GFP and PIN3:GFP fusion proteins, anti-GFP antibody (Abcam) was used for the detection of GFP-tagged fusion proteins. Coomassie Brilliant Blue-stained protein samples are shown as loading controls.
Phenotypic Analysis of Transgenic Plants, Mutants, and Gravity Response
Seedlings of wild-type, transgenic plants and mutants were grown on half-strength MS medium containing 1.5% sucrose and 0.8% agar for 4 d, then transferred to medium supplemented with different concentrations of NAA (0, 50, 75, and 100 nM and 1 μM) for another 5 d, and the primary root length was measured. For hypocotyl elongation analysis, seedlings were grown at 22 or 29°C in the vertical position for 7 d, and hypocotyl lengths were measured. The root gravity response was determined using 4-d-old seedlings. The angle of root curvature was measured at 2, 4, 8, 12, and 24 h after a 90° reorientation. The above measurements were performed with the aid of the image analysis software Image J (version 1.47; http://rsb.info.nih.gov/ij). Thirty to fifty seedlings were used for measurement, and data presented are the averages of 30 to 50 seedlings with sd. The statistical significance was evaluated by Student’s t test analysis. For multiple comparisons, an analysis of variance followed by Dunnett’s LSD method was performed on the data. Single and double asterisks indicate significant differences from control at the level of P < 0.05 and P < 0.01, respectively.
Pollen Phenotype Analysis of rop3 Mutants
Freshly anther-dehisced flowers were used for pollen viability assays and in vivo pollen germination experiments. Pollen grains from the dehisced anthers of randomly picked flowers were dipped in the Alexander and 4′,6-diamidino-2-phenylindole solution droplets on slides and observed by differential interference contrast and fluorescence microscopy, respectively. The limited pollination experiments were performed as described by Chen et al. (2007). Stage 12 flowers were emasculated and pollinated by 10 to 30 pollen grains from wild-type and rop3 mutant plants 12 h after emasculation. Pistils were collected 2 h after pollination. Aniline blue staining of pollen tubes was performed as described by Chen et al. (2007).
Marker Gene Analysis
DR5rev:GFP, PIN1pro:PIN1:GFP, PIN2pro:PIN2:GFP, PIN3pro:PIN3:GFP, AUX1pro:AUX1:YFP, PLT1pro:PLT1:YFP, PLT2pro:PLT2:YFP, and Mppro:n3xGFP were individually crossed into rop3-1 and the strongest transgenic line of DN-rop3. Homozygous plants were isolated from F2 populations. These homozygotes in F3 and later generations were used for analyses.
BFA Treatment and Washout Analysis
Three-day-old seedlings were incubated in half-strength MS medium containing 100 μM BFA (Sigma-Aldrich) for 40 min and observed or washed with half-strength MS medium for 90 min. For quantitative analysis of BFA bodies, stele cells from 15 to 30 roots were used for each assay.
MG132 Treatment and Lysotracker Red Labeling
Five-day-old seedlings were incubated with 50 μM MG132 (Sigma-Aldrich) for 4 h in darkness, and DMSO incubation was used as the control. Lysotracker red (2 μM; Invitrogen) was applied on seedlings for 1 h before confocal imaging analysis.
Microscopy
Ovules and seedling roots were cleared in a Hoyer’s solution and an HCG (water, chloral hydrate, and glycerol-containing) solution as previously described (Chen et al., 2011). For Lugol staining of roots, root tips were incubated in a 1:1 dilution of Lugol’s solution (Sigma-Aldrich) for 1 min, then washed with water and mounted in the HCG solution for microscopy analysis. Histochemical staining for GUS activity was performed as previously described (Chen et al., 2011). Embryos, different tissues, and seedlings were incubated in GUS (0.5 mg/mL) staining buffer from 6 to 36 h at 37°C. Differential interference contrast imaging was performed on an Olympus BX51 microscope equipped with a Ritiga 2000R camera. Seedlings were photographed under a Nikon SMZ1000 microscope using a Nikon digital sight Ds-Fi1 camera.
Dissected embryos and seedlings were stained with 1 μg/mL FM4-64 and 100 μg/mL propidium iodide for confocal microscopy. GFP, YFP, FM4-64, and propidium iodide fluorescence were imaged under SP2 Leica confocal laser scanning microscope. The fluorescent markers were visualized with the following excitation (Ex) and emission (Em) wavelengths (Ex/Em): 488 nm/505 to 530 nm for GFP, 514 nm/530 to 560 nm for YFP, 543 nm/600 nm for FM4-64, and 561 nm/591 to 635 nm for propidium iodide.
Twenty to thirty samples for each line were first analyzed under the Olympus BX51 microscope and then 10 to 15 samples were used for confocal imaging. Each experiment was repeated three to four times with similar results.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ACTIN2 (At3g18780), PIN1 (At1g73590), PIN2 (At5g57090), PIN3 (At1g70940), AUX1 (At2g38120), PIP2 (At3g53420), MP (At1g19850), PLT1 (At3g20840), PLT2 (At1g51190), RopGEF7(At5g02010), and ROP3 (At2g17800).
Supplemental Data
The following materials are available in the online version of the article.
Supplemental Figure 1. Analysis of ROP3 Expression Pattern and Subcellular Localization.
Supplemental Figure 2. qRT-PCR Analysis of DN-rop3 Transcripts and Immunoblot Analysis of DN-rop3 Protein Levels in the DN-rop3 Transgenic Lines.
Supplemental Figure 3. Identification of the rop3 T-DNA Insertion Mutants.
Supplemental Figure 4. The ROP3pro:ROP3 Transgene Rescues the Phenotypes in rop3 Mutants.
Supplemental Figure 5. Pollen Development in rop3 Mutants Is Normal.
Supplemental Figure 6. ROP3 Is Required for the Accumulation of PIN1pro:PIN1:GFP and PIN3pro:PIN3:GFP.
Supplemental Figure 7. ROP3 Has No Effect on the Transcript Levels of PINs and AUX1 Genes.
Supplemental Figure 8. ROP3 Does Not Affect the Localization of PIN2:GFP.
Supplemental Figure 9. RopGEF7 Does Not Affect the Polarity of PIN1 and PIN3.
Supplemental Figure 10. ROP3 Does Not Affect the Trafficking of PIN2:GFP in Roots.
Supplemental Figure 11. ROP3 Does Not Affect the Trafficking of PIP2:GFP in Roots.
Supplemental Figure 12. PIN3:GFP Does Not Accumulate in Vacuole Compartments in MG132-Treated Roots of DN-rop3 and rop3.
Supplemental Table 1. Primers Used in This Study.
Supplemental Table 2. Quantitative Analysis of DN-rop3 and rop3 Embryonic Phenotypes.
Supplemental Table 3. Quantitative Analysis of DN-rop3 and rop3 Seedling Phenotypes.
Supplemental Table 4. Quantitative Analysis of Embryo Phenotypes in the ROP3pro:ROP3 Transgene Rescued rop3 Background.
Supplementary Material
Acknowledgments
We thank the ABRC (Ohio State University) and Ben Scheres, Chuanyou Li, Dolf Weijers, Jianwei Pan, and Malcolm Bennett for providing seeds used in this study. We thank Hen-ming Wu for critical reading and suggestions for the article. We thank Weicai Yang and Yanjiao Zou for sharing the protocols for pollen assays with us. This work was supported by grants from National Natural Science Foundation of China (91117007 and 31370312) and the Ministry of Science and Technology of China (2007CB948200). J.X. was supported by the Ministry of Education of Singapore Academic Research Fund (Tier 2; MOE2012-T2-1-157).
AUTHOR CONTRIBUTIONS
J.-b.H., H.L., M.C., J.X., A.Y.C., and L.-z.T. designed the research. J.-b.H., H.L., M.C., X.L., M.W., Y.Y., C.W., J.H., G.L., and Y.L. performed the experiments. J.-b.H., H.L., M.C., J.X., A.Y.C., and L.-z.T. analyzed the data. L.-z.T. wrote the article. A.Y.C., J.X., and L.-z.T. revised the article.
Glossary
- PM
plasma membrane
- qRT-PCR
quantitative RT-PCR
- RNAi
RNA interference
- BFA
brefeldin A
- MS
Murashige and Skoog
- NAA
1-naphthaleneacetic acid
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Aida, M., Beis, D., Heidstra, R., Willemsen, V., Blilou, I., Galinha, C., Nussaume, L., Noh, Y.S., Amasino, R., Scheres, B. (2004). The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120 [DOI] [PubMed] [Google Scholar]
- Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jürgens, G., Friml, J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., Feldmann, K.A. (1996). Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Berken, A., Thomas, C., Wittinghofer, A. (2005). A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436: 1176–1180 [DOI] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., Scheres, B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Chen, M., Liu, H., Kong, J., Yang, Y., Zhang, N., Li, R., Yue, J., Huang, J., Li, C., Cheung, A.Y., Tao, L.Z. (2011). RopGEF7 regulates PLETHORA-dependent maintenance of the root stem cell niche in Arabidopsis. Plant Cell 23: 2880–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., Naramoto, S., Robert, S., Tejos, R., Löfke, C., Lin, D., Yang, Z., Friml, J. (2012). ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr. Biol. 22: 1326–1332 [DOI] [PubMed] [Google Scholar]
- Chen, Y.H., Li, H.J., Shi, D.Q., Yuan, L., Liu, J., Sreenivasan, R., Baskar, R., Grossniklaus, U., Yang, W.C. (2007). The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19: 3563–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S.K., Dagenais, N., Chory, J., Weigel, D. (2000). Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Clough, S.J., Bent, A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Craddock, C., Lavagi, I., Yang, Z. (2012). New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol. 22: 492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S.R., Ehrhardt, D.W., Griffitts, J.S., Somerville, C.R. (2000). Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe, P., Huang, F., Galvan-Ampudia, C.S., Mähönen, A.P., Kleine-Vehn, J., Xu, J., Quint, A., Prasad, K., Friml, J., Scheres, B., Offringa, R. (2010). Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137: 3245–3255 [DOI] [PubMed] [Google Scholar]
- Dinneny, J.R., Long, T.A., Wang, J.Y., Jung, J.W., Mace, D., Pointer, S., Barron, C., Brady, S.M., Schiefelbein, J., Benfey, P.N. (2008). Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Duan, Q., Kita, D., Li, C., Cheung, A.Y., Wu, H.M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig, L.A. (1999). Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1: E25–E27 [DOI] [PubMed] [Google Scholar]
- Foreman, J., Demidchik, V., Bothwell, J.H., Mylona, P., Miedema, H., Torres, M.A., Linstead, P., Costa, S., Brownlee, C., Jones, J.D., Davies, J.M., Dolan, L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Friml, J., Vieten, A., Sauer, M., Weijers, D., Schwarz, H., Hamann, T., Offringa, R., Jürgens, G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Friml, J., Wiśniewska, J., Benková, E., Mendgen, K., Palme, K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Friml, J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Galinha, C., Hofhuis, H., Luijten, M., Willemsen, V., Blilou, I., Heidstra, R., Scheres, B. (2007). PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Geldner, N., Anders, N., Wolters, H., Keicher, J., Kornberger, W., Muller, P., Delbarre, A., Ueda, T., Nakano, A., Jürgens, G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Jürgens, G., Palme, K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gray, W.M., Ostin, A., Sandberg, G., Romano, C.P., Estelle, M. (1998). High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieneisen, V.A., Xu, J., Marée, A.F., Hogeweg, P., Scheres, B. (2007). Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013 [DOI] [PubMed] [Google Scholar]
- Gu, Y., Vernoud, V., Fu, Y., Yang, Z. (2003). ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J. Exp. Bot. 54: 93–101 [DOI] [PubMed] [Google Scholar]
- Hazak, O., Bloch, D., Poraty, L., Sternberg, H., Zhang, J., Friml, J., Yalovsky, S. (2010). A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 8: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo, W.D., Inoue, T., Park, W.S., Kim, M.L., Park, B.O., Wandless, T.J., Meyer, T. (2006). PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais, Y., Santambrogio, M., Rozier, F., Fobis-Loisy, I., Miège, C., Gaude, T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Kaothien, P., Ok, S.H., Shuai, B., Wengier, D., Cotter, R., Kelley, D., Kiriakopolos, S., Muschietti, J., McCormick, S. (2005). Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 42: 492–503 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn, J., Dhonukshe, P., Sauer, M., Brewer, P.B., Wiśniewska, J., Paciorek, T., Benková, E., Friml, J. (2008b). ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 18: 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn, J., Leitner, J., Zwiewka, M., Sauer, M., Abas, L., Luschnig, C., Friml, J. (2008a). Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C., Chua, N.H. (1999). Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, S., Slane, D., Herud, O., Kong, J., Jürgens, G. (2012). Early embryogenesis in flowering plants: setting up the basic body pattern. Annu. Rev. Plant Biol. 63: 483–506 [DOI] [PubMed] [Google Scholar]
- Laxmi, A., Pan, J., Morsy, M., Chen, R. (2008). Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE 3: e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Lin, Y., Heath, R.M., Zhu, M.X., Yang, Z. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11: 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, D., et al. (2012). A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr. Biol. 22: 1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfke, C., Luschnig, C., Kleine-Vehn, J. (2013). Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech. Dev. 130: 82–94 [DOI] [PubMed] [Google Scholar]
- Michniewicz, M., et al. (2007). Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Nagawa, S., Xu, T., Lin, D., Dhonukshe, P., Zhang, X., Friml, J., Scheres, B., Fu, Y., Yang, Z. (2012). ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 10: e1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau, C., Cheung, A.Y. (2011). New insights into the functional roles of CrRLKs in the control of plant cell growth and development. Plant Signal. Behav. 6: 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau, C., Wu, H.M., Cheung, A.Y. (2006). RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 11: 309–315 [DOI] [PubMed] [Google Scholar]
- Petricka, J.J., Winter, C.M., Benfey, P.N. (2012). Control of Arabidopsis root development. Annu. Rev. Plant Biol. 63: 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher, E.H., Lokerse, A.S., Schlereth, A., Llavata-Peris, C.I., Bayer, M., Kientz, M., Freire Rios, A., Borst, J.W., Lukowitz, W., Jürgens, G., Weijers, D. (2012). Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 22: 211–222 [DOI] [PubMed] [Google Scholar]
- Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C.L., Paris, S., Gälweiler, L., Palme, K., Jürgens, G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Swarup, K., et al. (2008). The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup, R., et al. (2004). Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, L.Z., Cheung, A.Y., Wu, H.M. (2002). Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, L.Z., Cheung, A.Y., Nibau, C., Wu, H.M. (2005). RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell 17: 2369–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas, A., Paque, S., Stierlé, V., Quettier, A.L., Muller, P., Lechner, E., Genschik, P., Perrot-Rechenmann, C. (2013). Auxin-binding protein 1 is a negative regulator of the SCF(TIR1/AFB) pathway. Nat. Commun. 4: 2496. [DOI] [PubMed] [Google Scholar]
- Vieten, A., Vanneste, S., Wisniewska, J., Benková, E., Benjamins, R., Beeckman, T., Luschnig, C., Friml, J. (2005). Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Weijers, D., Franke-van Dijk, M., Vencken, R.J., Quint, A., Hooykaas, P., Offringa, R. (2001). An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128: 4289–4299 [DOI] [PubMed] [Google Scholar]
- Wisniewska, J., Xu, J., Seifertová, D., Brewer, P.B., Ruzicka, K., Blilou, I., Rouquié, D., Benková, E., Scheres, B., Friml, J. (2006). Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Wu, H.M., Hazak, O., Cheung, A.Y., Yalovsky, S. (2011). RAC/ROP GTPases and auxin signaling. Plant Cell 23: 1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., Wen, M., Nagawa, S., Fu, Y., Chen, J.G., Wu, M.J., Perrot-Rechenmann, C., Friml, J., Jones, A.M., Yang, Z. (2010). Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky, S., Bloch, D., Sorek, N., Kost, B. (2008). Regulation of membrane trafficking, cytoskeleton dynamics, and cell polarity by ROP/RAC GTPases. Plant Physiol. 147: 1527–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. (2002). Small GTPases: versatile signaling switches in plants. Plant Cell 14 (suppl.): S375–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Fu, Y. (2007). ROP/RAC GTPase signaling. Curr. Opin. Plant Biol. 10: 490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti, H., Anthony, R.G., Jahchan, N., Bögre, L., Christensen, S.K. (2006). Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 6404–6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D., Wengier, D., Shuai, B., Gui, C.P., Muschietti, J., McCormick, S., Tang, W.H. (2008). The pollen receptor kinase LePRK2 mediates growth-promoting signals and positively regulates pollen germination and tube growth. Plant Physiol. 148: 1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., McCormick, S. (2007). A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 18830–18835 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.