Abstract
Antimicrobial peptides (AMPs) are an essential part of innate immunity. These compounds have been considered as potential therapeutics because of their broad-spectrum activities and proven ability to avoid antimicrobial resistance, but their clinical and commercial developments have some limitations, such as susceptibility to proteases and a high cost of peptide production. To overcome these problems, many researchers have tried to develop short active peptides, their modifications and mimics with better properties while retaining their basic features of natural AMPs such as cationic charge and the amphipathic structure.
Keywords: antimicrobial peptides, antibacterial activity, peptide analogs, peptidomimetics, peptoids
Introduction
Antimicrobial peptides (AMPs), also called host defense peptides, are biologically active molecules produced by a wide variety of organisms as an essential component of their innate immune response.1 The major part of AMPs is formed from high molecular precursors as a result of post-translational modification.2 AMPs can be classified according to their structure, origin, biosynthesis mechanism, localization, biological function, mechanism of action, activity, and specificity. In the vast majority of cases, AMPs have the cationic structure1,3 but anionic AMPs have also been described in literature.4 Based on their structures, AMPs can be grouped into four classes: α-helical peptides, β-sheet peptides, extended peptides, and loop peptides.5 The α-helical AMPs constitute a representative class of antibacterial peptides that have the most well-determined structure–activity relationships. Lack of cysteine residues in the molecule is characteristic of peptides of this group. These peptides have a disordered structure in aqueous solutions, but in the presence of trifluoroethanol or liposomes, these molecules are converted into α-helix forms.2 The β-sheet AMPs are stabilized by disulfide bridges and form relatively rigid structures. The extended AMPs, which are predominantly rich in specific amino acids, such as proline, tryptophan, arginine, and histidine, have no regular secondary structure elements. The loop AMPs adopt a loop formation with one disulfide bridge (Table 1). AMPs show great structural diversity, but some features common to most of them are: a relatively small size (generally between 12 and 50 amino acid residues), their cationic nature because of multiple Arg or/and Lys residues, and the amphipathic structure because of the presence of both hydrophobic and hydrophilic regions.
Table 1.
Examples of antibacterial peptides isolated from different sources.
| PEPTIDES | ORIGIN | NUMBRR OF AMINO ACIDS IN MOLECULE | LITERATURE |
|---|---|---|---|
| α-helical | |||
| magainin 2 | frog | 23 | 6 |
| cecropin A | insects | 37 | 7 |
| LL-37 (cathelicidin) | human | 37 | 8 |
|
| |||
| β-sheet | |||
| α-defensins and β-defensins (containing three disulphide bonds) | human | 29–35 and 36–47 | 9, 10 |
| protegrin 1 (containing two disulphide bonds) | pig | 18 | 11, 12 |
|
| |||
| extended structure | |||
| indolicin (tryptophan and proline rich peptide) | bovine | 13 | 13 |
|
| |||
| Loop | |||
| (containing one disulphide bond) | |||
| Bactenecin | bovine | 12 | 14 |
| Lactoferricin | bovine | 25 | 15 |
Mechanism of Action
AMPs show a broad spectrum of antimicrobial activities against various microorganisms. Many of these peptides are effective against multidrug-resistant bacteria and possess a low propensity for developing resistance.
The precise mechanism of the action of AMPs is yet to be explained. The electrostatic interaction of cationic AMPs with negatively charged molecules on the membrane of bacteria cells seems to be the primary mechanism for antimicrobial activity. Currently, there are three most popular models explaining the increase in membrane permeability because of AMP action: the barrel-stave model, the toroidal pore model, and the carpet model (Fig. 1).16–19 According to the barrel-stave model, a variable number of channel-forming peptides are positioned in a “barrel-like” ring around an aqueous pore. In the toroidal pore model, the peptides induce membrane depolarization and form toroidal-shaped transmembrane pores. According to the carpet model, the peptides first bind to the target membrane and cover it in a “carpet-like” manner, then they disintegrate the membrane by disrupting the bilayer curvature.
Figure 1.
Three most popular modes of action of antimicrobial peptides against cytoplasmic membranes. (A) Barrel-stave model; (B) carpet model (C) toroidal pore model according to Ref. 19.
Owing to a high level of cholesterol and low anionic charge, eukaryotic cells become out of the target range of many AMPs.20 Understanding the selectivity of different AMPs for mammalian and bacterial membranes is of obvious interest in the development of these peptides as novel antibacterial agents. Molecular dynamic simulations are used to visualize in detail the interactions between AMPs and a variety of membrane mimics. This helps to understand the molecular mechanisms of the antimicrobial activity of these compounds and their toxicity.21–23 Several reviews connected with the structures, potential mechanisms of action, and biological activities of AMPs have recently been published.24–34
The AMPs have been considered promising and potential drug candidates for the future because of their broad range of activity, lesser toxicity, and decreased resistance development by their target cells.
Structure Modification
The widespread use of antibiotics has led to the development of numerous multidrug resistant strains, resulting in an urgent need to develop new effective antimicrobial agents capable of being established as therapies for bacterial infections. The AMPs have a proven ability to avoid antimicrobial resistance, a phenomenon that has rendered many pharmacologically derived antibiotics ineffective. Probably one advantage of AMPs is the generality of their mechanism of action, which involves either breaking the bacterial membrane integrity or disrupting their essential components inside their cells.35,36 This differs from the specific receptors targeted by conventional antibiotics, which allows the pathogenic bacteria to develop resistance more rapidly. AMPs are also fast-acting and potent compounds. They can be metabolically and rapidly cleared from the body. Although AMPs possess considerable benefits, their clinical and commercial developments have some limitations. The disadvantage of the AMPs is the fact that most peptides cannot be administered orally as they are rapidly inactivated by gastrointestinal enzymes, so that subcutaneous or intravenous administration is required.37–39 Although peptides exhibit significant in vitro activity against bacteria, for many peptides this activity appears to be lost under physiological salt and serum conditions. Salt-dependent inactivation of human β-defensins is an example.40 Another problem is the size of molecules. They are small in comparison with proteins but large from the point of view of the chemical peptide synthesis. This is connected with high costs of their production. The price of synthetic peptides is considerably higher in comparison with conventional antibiotics. It can be a reason that the pharmaceutical industry has been reluctant to promote the clinical use of this class of antibacterial therapeutics.33,40 Because of this problem, the investigations have been centered on searching for smaller peptides with antimicrobial activity.
Lactoferrin Derivatives
An example of an approach to this problem was the investigation of short fragments of complex AMP, lactoferrin or its 25-residue N-terminal fragment, lactoferricin41 (Fig. 2), for establishing minimal structural requirements for antimicrobial activity (Table 2).42–48 An 11-residue linear peptide portion of bovine lactoferricin has been reported to have similar antimicrobial activity to lactoferricin itself, but with lower hemolytic activity.47 The synthetic AMP hLF1–11, derived from the first 11 amino acids of human lactoferrin, was evaluated in both preclinical and clinical trials (Table 3), and it is an interesting candidate for further exploration.49 The antibacterial active fragment of bovine lactoferricin, hexapeptide RRWQWR, was determined by Tomita et al.42 Many fragments of lactoferricin were synthesized and examined,43–50 from the 15 residue peptide LFB, consisted of 17–31 residues of bovine lactoferrin,43 to the modified dipeptides.44 According to the pharmacophore model of short AMPs,51 cationic charged as well as bulky and lipophilic moieties are necessary in the structure of such compounds. Charged moieties generally consisted of a side chain of arginine or lysine. Bulk units were represented by an indol, phenol, or phenyl group. Other studies suggest that the antimicrobial activity of peptides containing arginine is higher than those of peptides containing lysine, while peptides containing tryptophan are more potent than those with either phenylalanine or tyrosine.
Figure 2.
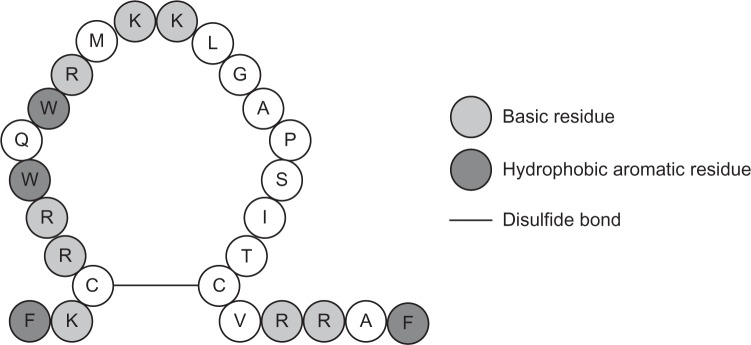
Amino acid sequence of cyclic bovine lactoferricin according to Ref. 46.
Table 2.
Antibacterial activity of lactoferricin and its truncated analogs, reported as minimal inhibitory concentration (MIC) according to Ref. 48.
| PEPTIDE | NUMBER OF AMINO ACIDS | MIC [μM] S. aureus | E. coli |
|---|---|---|---|
| Cyclic lactoferricin | 25 | 2–10 | 2–10 |
| Lactoferricin 1–15 | 15 | 48–150 | 20–14 |
| RWRNHBn | 3 | 124 | >150 |
| RBipRNHBn | 3 | 16 | >150 |
Abbreviation: Bip, 4,4′-biphenylalanine.
Table 3.
| NAME | NUMBER OF AMINO ACIDS | DESCRIPTION | INTENDED USE | COMPANY | TRIAL PHASE |
|---|---|---|---|---|---|
| Omiganan (MBI-226, MX-226, CSL-001) | 12 | Synthetic analog of indolicidin | Topical antiseptic prevention of catheter infection, severe acne and rosacea | Migenix/BioWest therapeutics Cutanea Life Sciences | III/II |
| Pexiganan (MSI-78) | 22 | Synthetic analog of magainin 2 | Topical antibiotic – diabetic ulcers | Macrochem | III |
| Iseganan (IB-367) | 17 | Protegrin 1 derivative | Prevention of oral mucositis | Ardea Biosciences | III |
| LTX-109 | 3 | Peptidomimetic | Topical antibiotic | Lytic Biopharma | I/II |
| hLF1–11 | 11 | Lactoferrin derivative | Prevention of bacteraemia and fungal infections | AM Pharma | I/II |
| OP-145 | 24 | LL-37 derivative | Treatment of chronic middle ear infection | Octoplus | I/II |
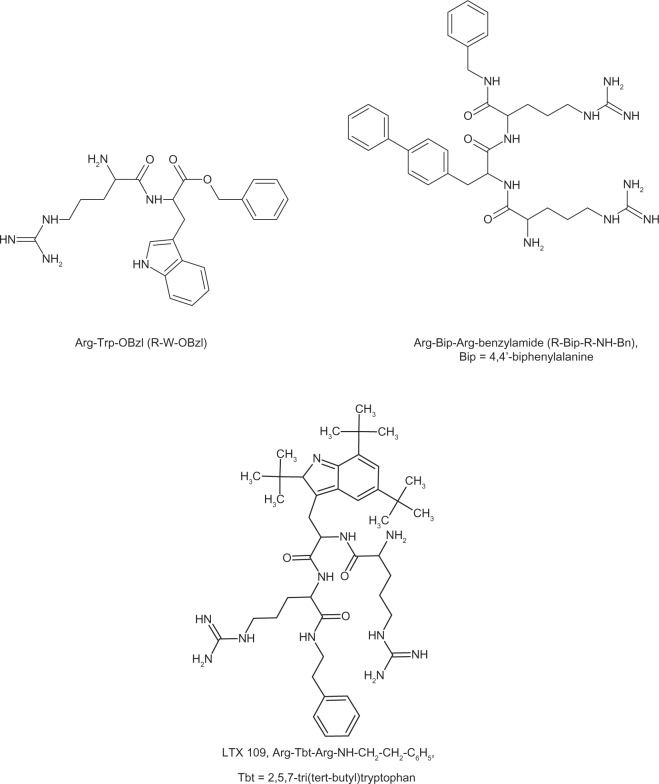
The series of peptides containing simple repeating sequence: (RW)n-NH2 (where n = 1–5) were synthesized, and their antimicrobial and hemolytic activity was determined. The hexapeptide (n = 3) represents an optimal chain length in terms of the efficacy of peptide synthesis and antibacterial activity and selectivity (evaluated by the hemolytic index).52 According to the authors,51 the minimal anti-staphylococcal motif can be defined as a combination of two bulky moieties and two charged groups. An example of such a peptide is RW-OBzl (Fig. 3), where the bulky units are the indole side chain of tryptophan and the benzyl ester group, and where the charged units are the guanidinium group of arginine and the free N-terminal amino group.51 To improve the properties of the synthesized peptides, different bulky aromatic amino acids were used instead of tryptophan.43,44,48,53 Replacing the indole side chain of Trp with bulkier, more hydrophobic groups has shown to yield peptides with increased antibacterial activity. Antimicrobial tripeptides with a modified structure such as l-arginyl-l-4,4′-biphenylalanyl-l-arginine benzylamide, where l-4,4′-biphenylalanine was used to replace tryptophan residue (Fig. 3, Table 2),48 belong to extremely truncated versions of lactoferrin. The modified tryptophan molecule (2,5,7-tri(tert-butyl)tryptophan-Tbt) was also applied in the synthesis of the modified tripeptides with general structure RTbtR-X, where X was –OMe, unsubstituted and substituted amides.54,55 The most promising structure seems to be the tripeptide with C-terminal amide substituted by the ethylphenyl group (LTX-109) (Fig. 3, Table 3).55
Figure 3.
Short peptides derived from lactoferrin with minimal antibacterial motif.
AMPs Derivatives
Another possibility to design small peptides with antimicrobial activity was using combinatorial libraries for the examination of potential short active peptide sequences.56–58 These libraries were not only used to discover novel short AMPs56 but also to improve the activity of already known compounds.58 The result of such investigations was the synthesis of arginine and tryptophan-rich hexapeptides called by the authors combi-1 (Ac-RRWWRF-NH2) and combi-2 (Ac-FRWWHR-NH2). These hexapeptides possess antibacterial activities similar to natural peptides.35 Smaller AMPs like cationic containing 13 amino acid peptide amide – indolicin59 or dodecapeptide – bactenecin60,61 were used as a lead structure to develop potential new antimicrobial drugs, for example, omiganan (ILRWPWWPWRRK-NH2) (Table 3), a synthetic cationic peptide derived from indolicin.62,63 To obtain short cationic peptides with enhanced antimicrobial activity, fluorine atoms or trifluoromethyl groups were introduced in the structure.64 Another reason for the structure modification of AMPs is searching for compounds with a potential for oral administration. It is connected with the problem of their stability toward the enzymatic degradation. Series of short cationic peptides (generally, tripeptides), with the presence of unnatural amino acids in the structure, were synthesized by the application of combinatorial libraries and its antibacterial activity, and their stability for tryptic65,66 and chymotryptic67 degradation was examined. The use of Trp and Arg analogs can minimize the interaction of antimicrobial tripeptides with binding pockets of proteolytic enzymes and increase their stability.48 The replacement of arginine with Agp (α-amino-3-guanidino-propionic acid) in AMP Sub-3 (NH2-RRWRIVVIRVRR-CONH2) protects this compound from its fast degradation in serum.68 Also the cyclization of short tryptophan and arginine-rich AMPs generally brings about significant stabilization against serum proteases.50
Another Antibacterial Compounds with Peptide Structure
Antibacterial activity was also observed in the case of peptides that are not fragments or analogs of natural AMPs. Several compounds with the linear or cyclic dipeptide structure belong to this group. Antimicrobial dipeptide β-alanyl-tyrosine was isolated from insects.69 Series of antimicrobial analogs of Trp-His and His-Arg70 as well as dipeptide-based amphiphiles71 were synthesized. Antimicrobial cyclic dipeptides (diketopiperazines) were isolated from a Bacillus sp. strain.72,73 Peptide dendrimers – branched polymers with peptides attached centrally to the core matrix – were developed for a number of different applications.74 Antimicrobial activity was observed in the case of dendrimeric peptides containing a lysine core with attached two or eight copies of tetrapeptide R4 (RLYR) or octapeptide R8 (RLYRKVYG).75 Low molecular dendrimers containing basic amino acid lysine and hydrophobic fragments also showed antibacterial activity. According to the authors,76 such amphiphilic dendrimeric peptides can be non-sequential pharmacophores, which mimic the active conformation of linear AMPs. Lipopeptides are native antimicrobial agents produced in bacteria and fungi. They are composed of aliphatic fatty acid attached to N-terminus of a short peptide.74 While searching for new antibacterial compounds, a series of short lipopeptides constructed from natural l and d amino acids was synthesized.77,78 A non-genetically coded amino acid ornithine was also used to synthesize short peptides covalently attached to fatty acids of different chain lengths.79,80 The obtained results demonstrate a strong potential of lipopeptides as a class of novel antimicrobial therapeutics.80
Peptoids and Peptidomimetics
The antimicrobial β-peptides have been designed as oligomers mimicking the structure of AMP magainin.81 They can adopt a variety of different helical conformations and are resistant toward the degradation by trypsin and chymotrypsin.82 A series of short, highly potent β-peptidomimetics based on the pharmacophore model of short AMPs51 were obtained by coupling of achiral lipophilic 3-amino-2,2-disubstituted propionic acid to a C-terminal l-arginine amide residue.83
Oligoacyllysines (OAKs) are a group of antimicrobial compounds, composed of tandem repeats of acyllysines. These simple structures were designed to mimic the primary structure and function of natural AMPs. To study the structure–activity relationships in OAKs, the library composed of 103 OAKs have been synthesized. Only few members of this library displayed the ability to inhibit bacterial growth. Antimicrobial activity was observed in the case of short (dimer) dodecanoyl-based OAK.84 Further investigations suggested that the minimal requirements for potent and selective antibacterial activity with low hemolytic activity represent the sequence: aminolauryl-[lysyl-aminolauryl-lysyl]2 (designated NC12–2β12).85 Peptoids (N-substituted glycines) are mimics of α-peptides in which their side chains are attached to the backbone of Nα amide nitrogen instead of the Cα-atom.74,82,86 A series of peptoids constructed from N-alkylated glycine residues were synthesized as potential mimics of antimicrobial lipopeptides.87 The head-to-tail macrocyclization of linear peptoids was used to enhance the antimicrobial activity of these compounds.88 The combinations of the peptoid subunits with lysine residues were also investigated. Several such lysine–peptoid hybrids showed potent antibacterial and low hemolytic activities.89,90 For example, N,N-disubstituted l-lysine amides were the simplest peptoids with antimicrobial activity.91 Two positive charges were contributed by α and ε groups of l-lysine; the hydrophobicity was brought about by a substituent of amide nitrogen: alkyl chain and bulky aromatic core.
Clinical Development
In their search for new antimicrobial therapeutics, several pharmaceutical companies have been attempting to introduce AMPs into the market.29 Recent investigations generally focus on relatively small and cost-effective molecules that contain only the biologically active core region of the natural AMP. AMPs are in various stages of drug development, from early preclinical studies to phase III of clinical studies (Table 3). Compounds with different molecular sizes were examined, from the peptide containing 24 amino acids, OP-145,67 to the modified tripeptide LTX-109 (Fig. 3),55 both in phase II of clinical trial (Table 3). There has been limited success with those AMPs that were introduced into clinical trials, especially when the results were compared to conventional antibiotics.92,93 Two indolicin-based AMPs, MBI-226 and MX-594AN, which have been developed for the treatment of catheter-related infection and acne, respectively, belong to the most advanced potential therapeutics.23,29,62,63,92 MBI-226 (omiganan) has now completed two separate phase III clinical trials demonstrating safety and significant efficacy in decreasing catheter colonization and reducing microbiologically confirmed tunnel infections (Table 3).93 The other one has been less successful. The AMP – pexiganan – a broad-spectrum synthetic analog of the African frog peptide magainin, developed for the treatment of diabetic foot ulcers, was denied approval for clinical use.23,29 This compound did not demonstrate an advantage over existing fluoroquinolone therapy.23,29,92,93 But it has recently been announced that pexiganan would re-enter phase III trials to enable the resubmission of a new drug application. It has also been reported that the second generation of AMPs based on pexiganan have been developed.93 None of the AMPs have been granted Food and Drug Administration (FDA) approval for clinical use.23
Conclusions
Out of thousands of potential synthetic peptides with antimicrobial activity, only a small number have been systematically studied and tested. Many AMPs and its analogs have been synthesized. Some of them have very small molecules (di- and tripeptides) and improved stability against the enzyme digestion, so they are devoid of certain disadvantages of natural AMPs and can be more suitable for pharmaceutical applications. The field is young, and it would be premature to conclude if AMPs can be clinically used therapeutic agents. Many AMPs have demonstrated antimicrobial activity under controlled experimental conditions in vitro, but these results have not yet been transformed into success as therapeutics in clinical trials. Although some early preclinical studies as well as clinical trials have been encouraging, no AMP has yet been approved for clinical use. The question whether AMPs can be a new class of future drugs still remains unanswered.
Footnotes
ACADEMIC EDITOR: Yitzhak Tor, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived the concepts: KMN. Wrote the first draft of the manuscript: KMN, AM. Contributed to the writing of the manuscript: KMN, AM. Agree with manuscript results and conclusions: KMN, AM. Jointly developed the structure and arguments for the paper: KMN. Made critical revisions and approved final version: KMN, AM. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolimers (Peptide Science) 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw JP. Cationic antimicrobial peptides: issues for potential clinical use. BioDrugs. 2003;4:233–40. doi: 10.2165/00063030-200317040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harris F, Dennison SR, Phoenix DA. Anionic antimicrobial peptides from eukaryotic organisms. Curr Protein Pept Sci. 2009;10:585–606. doi: 10.2174/138920309789630589. [DOI] [PubMed] [Google Scholar]
- 5.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutics strategies. Nat Biotechnol. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 6.Bechinger B, Zasloff M, Opella SJ. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 1993;2:2077–84. doi: 10.1002/pro.5560021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestro L, Weiser JN, Axelsen PH. Antibacterial and antimembrane activities of Cecropin A in Escherichia coli. Antimicrob Agents Chemother. 2000;44:602–7. doi: 10.1128/aac.44.3.602-607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Jarczak J, Kościuczuk EM, Lisowski P, et al. Defensins: natural component of human innate immunity. Hum Immunol. 2013;74:1069–79. doi: 10.1016/j.humimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 10.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–47. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 11.Jang H, Ma B, Nussinov R. Conformational study of the protegrin-1 (PG-1) dimer interaction with lipid bilayers and its effect. BMC Struct Biol. 2007;7:21. doi: 10.1186/1472-6807-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaridis T, He Y, Prieto L. Membrane interactions and pore formation by the antimicrobial peptide protegrin. Biophys J. 2013;104:633–42. doi: 10.1016/j.bpj.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladokhin AS, Selsted MS, White SH. Bilayer interactions of indolicidin, a small antimicrobial peptide rich in tryptophan, proline, and basic amino acids. Biophys J. 1997;72:794–805. doi: 10.1016/s0006-3495(97)78713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M, Hancock REW. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Zhang TN, Yang W, Liu N. Effect of loop structure of bovine lactoferricin on apoptosis in Jurkat cells. Biometals. 2010;23:555–61. doi: 10.1007/s10534-010-9324-2. [DOI] [PubMed] [Google Scholar]
- 16.Yeaman MR, Yount NY. Mechanism of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LT, Haney E, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–70. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol. 2010;5:905–17. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S-C, Park Y, Hahm KS. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci. 2011;12:5971–92. doi: 10.3390/ijms12095971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langham A, Kaznessis YN. Molecular simulations of antimicrobial peptides. Methods Mol Biol. 2010;618:267–85. doi: 10.1007/978-1-60761-594-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mátyus E, Kandt C, Tieleman D. Computer simulation of antimicrobial peptides. Curr Med Chem. 2007;14:2789–98. doi: 10.2174/092986707782360105. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Schlamadinger DE, Kim JE, McCammon JA. Comparative molecular dynamics simulations of the antimicrobial peptide CM15 in model lipid bilayers. Biochim Biophys Acta. 2012;1818:1402–9. doi: 10.1016/j.bbamem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phoenix DA, Dennison SR, Harris F. Antibacterial Peptides. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2013. Antimicrobial peptides: their history, evolution, and functional promiscuity. [DOI] [Google Scholar]
- 25.Pushpanathan M, Gunasekaran P, Rajendhran J. Antimicrobial peptides: versatile biological properties. Int J Pept. 2013;2013:15. doi: 10.1155/2013/675391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsuji T, Gallo RL. Antimicrobial peptides: old molecules with new ideas. J Invest Dermatol. 2012;132:887–95. doi: 10.1038/jid.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Lazarev VN, Govorun VM. Antimicrobial peptides and their use in medicine. Appl Biochem Microbiol. 2010;46:804–14. [Google Scholar]
- 29.Kang SJ, Kim DH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides: their physicochemical properties and therapeutic application. Arch Pharm Res. 2012;35:409–13. doi: 10.1007/s12272-012-0302-9. [DOI] [PubMed] [Google Scholar]
- 30.Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides for therapeutic application: a review. Molecules. 2012;17:12276–86. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–47. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6:1543–75. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuliani A, Pirri G, Nicoletto SF. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent Eur J Biol. 2007;2(1):1–33. [Google Scholar]
- 34.Brandenburg L-O, Merres J, Albrecht L-J, Varoga D, Pufe T. Antimicrobial peptides: multifunctional drugs for different applications. Polymers. 2012;4:539–60. [Google Scholar]
- 35.Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structure and mechanism of action. Biochim Biophys Acta. 2006;1758:1184–202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nat Rev Microbiol. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 37.Sun L. Peptide-based drug development. Mod Chem Appl. 2013;1:e103. [Google Scholar]
- 38.Edwards CMB, Cohen MA, Bloom SR. Peptides as a drugs. Q J Med. 1999;92:1–4. doi: 10.1093/qjmed/92.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Craig DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–47. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 40.Marr AK, Gooderham WJ, Hancock REW. Antibacterial peptides for therapeutic use: obstacles and realistic look. Curr Opin Pharmacol. 2006;6:468–72. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–6. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 42.Tomita M, Takase M, Bellamy W, Shimamura S. A review: the active peptide of lactoferrin. Acta Paediatr Jpn. 1994;36:585–91. doi: 10.1111/j.1442-200x.1994.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 43.Haug BE, Skar ML, Svendsen JS. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferrin derivatives. J Pept Sci. 2001;7:425–32. doi: 10.1002/psc.338. [DOI] [PubMed] [Google Scholar]
- 44.Haug BE, Strøm MB, Svendsen JSM. The medicinal chemistry of short lactoferrin-based antibacterial peptides. Curr Med Chem. 2007;14:1–18. doi: 10.2174/092986707779313435. [DOI] [PubMed] [Google Scholar]
- 45.Vogel HJ, Schilbi DJ, Jing W, Lohmeier-Vogel EM, Epand RF, Epand RM. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem Cell Biol. 2002;80:49–63. doi: 10.1139/o01-213. [DOI] [PubMed] [Google Scholar]
- 46.Moriaty LC, Joannou CL, van den Berg JJM, Gorinsky B, Evans RW. Factors contributing to the potency of antimicrobial cationic peptides from the N-terminal region of human lactoferrin. FEMS Microbiol Lett. 2004;239:295–9. doi: 10.1016/j.femsle.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen LT, Schibli DJ, Vogel HJ. Structural studies and model membrane interaction of two peptides derived from bovine lactoferricin. J Pept Sci. 2005;11:379–89. doi: 10.1002/psc.629. [DOI] [PubMed] [Google Scholar]
- 48.Svenson J, Vergote V, Karstad R, Burvenich C, Svendsen JS, De Spiegeleer B. Metabolic fate of lactoferricin-based antimicrobial peptides: effect of truncation and incorporation of amino acid analogs on the in vitro metabolic stability. J Pharmacol Exp Ther. 2010;332:1032–9. doi: 10.1124/jpet.109.162826. [DOI] [PubMed] [Google Scholar]
- 49.Brouwer CP, Rahman M, Welling MM. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides. 2011;32:1953–63. doi: 10.1016/j.peptides.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SAJ, Vogel HJ. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS One. 2010;5(9):e12684. doi: 10.1371/journal.pone.0012684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strøm MB, Haug BE, Skar ML, Stensen W, Stiberg T, Svendsen JS. The pharmacophore of short cationic antibacterial peptides. J Med Chem. 2003;46:1567–70. doi: 10.1021/jm0340039. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Brady A, Young A, et al. Length effects in antimicrobial peptides of the (RW)n series. Antimicrob Agents Chemother. 2007;51:597–603. doi: 10.1128/AAC.00828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haug BE, Stensen W, Stiberg T, Svendsen JS. Bulky nonproteinogenic amino acids permit the design of very small and effective cationic antibacterial peptides. J Med Chem. 2004;47:4159–62. doi: 10.1021/jm049582b. [DOI] [PubMed] [Google Scholar]
- 54.Haug BE, Stensen W, Kalaaji M. Rekdal Ø, Svendsen JS. Synthetic antimicrobial peptidomimetics with therapeutic potential. J Med Chem. 2008;51:4306–14. doi: 10.1021/jm701600a. [DOI] [PubMed] [Google Scholar]
- 55.Isaksson J, Brandsdal BO, Engqvist M, Flaten GE, Svendsen JSM, Stensen W. A synthetic antimicrobial peptidomimetics (LTX 109): stereochemical impact on membrane disruption. J Med Chem. 2011;54:5786–95. doi: 10.1021/jm200450h. [DOI] [PubMed] [Google Scholar]
- 56.Blondelle SE, Takahashi E, Weber PA, Houghten RA. Identification of antimicrobial peptides by using combinatorial libraries made up of unnatural aminoacids. Antimicrob Agents Chemother. 1994;38:2280–6. doi: 10.1128/aac.38.10.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blondelle SE, Takahashi E, Dingh KT, Houghten RA. The antimicrobial activity of hexapeptides derived from synthetic combinatorial libraries. J Appl Bacterol. 1995;78:39–46. doi: 10.1111/j.1365-2672.1995.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 58.Blondelle SE, Takahashi E, Houghten RA, Pérez-Payá E. Rapid identification of compounds with enhanced antimicrobial activity by using conformationally defined combinatorial libraries. Biochem J. 1996;313:141–8. doi: 10.1042/bj3130141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staubitz P, Peschel A, Nieuwenhuizen WF, et al. Structure-function relationship in the tryptophan rich, antimicrobial peptide indolicidin. J Pept Sci. 2001;7:552–64. doi: 10.1002/psc.351. [DOI] [PubMed] [Google Scholar]
- 60.Hilpert K, Elliott MR, Volkmer-Engert R, et al. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem Biol. 2006;13:1101–7. doi: 10.1016/j.chembiol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Hai Nan Y, Jacob B, Kim Y, Yub Shin S. Linear bactenecin analogs with cell selectivity and anti-endotoxic activity. J Pept Sci. 2012;18:740–7. doi: 10.1002/psc.2460. [DOI] [PubMed] [Google Scholar]
- 62.Melo MN, Castanho MARB. Omiganan interaction with bacterial membranes and cell wall models. Assigning a biological role to saturation. Biochim Biophys Acta. 2007;1768:1277–90. doi: 10.1016/j.bbamem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Rubinchik E, Dugourd D, Algara T, Pasetka C, Friedland HD. Antimicrobial and antifungal activities of a novel cationic antimicrobial peptide, omiganan, in experimental skin colonisation models. Int J Antimicrob Agents. 2009;34:457–61. doi: 10.1016/j.ijantimicag.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Giménez D, Andreu C, del Olmo M, Varea T, Diaz D, Asensio G. The introduction of fluorine atoms or trifluoromethyl groups in short cationic peptides enhances their antimicrobial activity. Bioorg Med Chem. 2006;14:6971–8. doi: 10.1016/j.bmc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 65.Svenson J, Stensen W, Brandsdal B-O, Haug BE, Monrad J, Svendsen JS. Antimicrobial peptides with stability towards tryptic degradation. Biochemistry. 2008;47:3777–88. doi: 10.1021/bi7019904. [DOI] [PubMed] [Google Scholar]
- 66.Karstad R, Isaksen G, Wynendaele E, et al. Targeting the S1 and S3 subsite of trypsin with unnatural cationic amino acids generates antimicrobial peptides with potential for oral administration. J Med Chem. 2012;55:6294–305. doi: 10.1021/jm3002058. [DOI] [PubMed] [Google Scholar]
- 67.Karstad R, Isaksen G, Brandsdal B-O, Svendsen JS, Svenson J. Unnatural amino acid side chains as S1,S1’, and S2’ probes yield cationic antimicrobial peptides with stability toward chymotrypic degradation. J Med Chem. 2010;53:5558–66. doi: 10.1021/jm1006337. [DOI] [PubMed] [Google Scholar]
- 68.Knappe D, Henklein P, Hoffman R, Hilpert K. Easy strategy to protect antimicrobial peptides from fast degradation in serum. Antimicrob Agents Chemother. 2010;54:4003–5. doi: 10.1128/AAC.00300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meylaers K, Cerstiaens A, Vierstraete E, et al. Antimicrobial compounds of low molecular mass are constitutively present in insects: characterization of β-alanyl-tyrosine. Curr Pharm Des. 2003;9:159–74. doi: 10.2174/1381612033392279. [DOI] [PubMed] [Google Scholar]
- 70.Sharma RK, Reddy RP, Tegge W, Jain R. Discovery of Trp-His and His-Arg analogues as new structural classes of short antimicrobial peptides. J Med Chem. 2009;52:7421–31. doi: 10.1021/jm900622d. [DOI] [PubMed] [Google Scholar]
- 71.Mitra RN, Shome A, Paul P, Das PK. Antimicrobial activity, biocompatibility and hydrogelation ability of dipeptide-based amphiphiles. Org Biomol Chem. 2009;7:94–102. doi: 10.1039/b815368j. [DOI] [PubMed] [Google Scholar]
- 72.Nishanth Kumar S, Mohandas C, Siji JV, Rajasekharan KN, Nambisan B. Identification of antimicrobial compound, diketopiperazines, from Bacillus sp. N strain associated with rhabditid entomopathogenic nematode against major plant pathogenic fungi. J Appl Microbiol. 2012;113:914–24. doi: 10.1111/j.1365-2672.2012.05385.x. [DOI] [PubMed] [Google Scholar]
- 73.Nishanth Kumar S, Dileep C, Mohandas C, Nambisan B, Jayaprakas CA. Cyclo (d-Tyr-d-Phe): a new antibacterial, anticancer, and antioxidant cyclic dipeptide from Bacillus sp.N strain associated with rhabditid entomopathogenic nematode. J Pept Sci. 2014;20:173–85. doi: 10.1002/psc.2594. [DOI] [PubMed] [Google Scholar]
- 74.Guliani A, Rinaldi AC. Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches. Cell Mol Life Sci. 2011;68:2255–66. doi: 10.1007/s00018-011-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tam JP, Lu Y-A, Yang JL. Antimicrobial dendrimeric peptides. Eur J Biochem. 2002;269:923–32. doi: 10.1046/j.0014-2956.2001.02728.x. [DOI] [PubMed] [Google Scholar]
- 76.Janiszewska J, Urbańczyk-Lipkowska Z. Amphiphilic dendrimeric peptides as model non-sequential pharmacophores with antimicrobial properties. J Mol Microbiol Biotechnol. 2007;13:220–5. doi: 10.1159/000104751. [DOI] [PubMed] [Google Scholar]
- 77.Avrahami D, Shai Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to D,L amino acids-containing antimicrobial peptides: a plausible mode of action. Biochemistry. 2003;42:14946–56. doi: 10.1021/bi035142v. [DOI] [PubMed] [Google Scholar]
- 78.Kamysz W, Silvestri C, Cirioni O, et al. In vitro activities of the lipopeptides palmitoyl (Pal)-Lys-Lys-NH2 and Pal-Lys-Lys alone and in combination with antimicrobial agents against multiresistant gram-positive Cocci. Antimicrob Agents Chemother. 2007;51:354–8. doi: 10.1128/AAC.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laverty G, McLaughlin M, Shaw C, Gorman SP, Gilmore BF. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem Biol Drug Des. 2010;75:563–9. doi: 10.1111/j.1747-0285.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 80.Lohan S, Cameotra SS, Bisht GS. Systematic study of non-natural short cationic lipopeptides as novel broad-spectrum antimicrobial agents. Chem Biol Drug Des. 2013;82:557–66. doi: 10.1111/cbdd.12182. [DOI] [PubMed] [Google Scholar]
- 81.Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. Antibiotics: non-haemolytic β-amino-acid oligomers. Nature. 2000;404:565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 82.Godballe T, Nilsson LL, Petersen PD, Jenssen H. Antimicrobial β-peptides and α-peptoids. Chem Biol Drug Des. 2011;77:107–16. doi: 10.1111/j.1747-0285.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 83.Hansen T, Alst T, Havelkova M, Strøm MB. Antimicrobial activity of small β-peptidomimetics based on the pharmacophore model of short cationic antimicrobial peptides. J Med Chem. 2010;53:595–606. doi: 10.1021/jm901052r. [DOI] [PubMed] [Google Scholar]
- 84.Radzishevsky IS, Kovachi T, Porat Y, et al. Structure relationships of antibacterial acyl-lysine oligomers. Chem Biol. 2008;15:354–62. doi: 10.1016/j.chembiol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Zaknoon F, Sarig H, Rotem S, et al. Antibacterial properties and mode of action of a short acyl-lysyl oligomer. Antimicrob Agents Chemother. 2009;53:3422–9. doi: 10.1128/AAC.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dohm MT, Kapoor R, Barron AE. Peptoids: bio-inspired polymers as potential pharmaceuticals. Curr Pharm Des. 2011;17(25):2732–47. doi: 10.2174/138161211797416066. [DOI] [PubMed] [Google Scholar]
- 87.Chongsiriwatana NP, Miller TM, Wetzler M, et al. Short alkylated peptoid mimics of antimicrobial lipopeptides. Antimicrob Agents Chemother. 2011;55:417–20. doi: 10.1128/AAC.01080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang ML, Shin SB, Benson MA, Torres VJ, Kirshenbaum K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem. 2012;7:114–22. doi: 10.1002/cmdc.201100358. [DOI] [PubMed] [Google Scholar]
- 89.Rynge TS, Hansen PR. Novel lysine-peptoid hybrids with antibacterial properties. J Pept Sci. 2005;11:727–34. doi: 10.1002/psc.705. [DOI] [PubMed] [Google Scholar]
- 90.Ryge TS, Hansen PR. Potent antibacterial lysine-peptoid hybrids identified from a positional scanning combinatorial library. Bioorg Med Chem. 2006;14:4444–51. doi: 10.1016/j.bmc.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh C, Manjunath GB, Akkapeddi P, et al. Small molecular antibacterial peptoid mimics: the simpler the better. J Med Chem. 2014;57:1428–36. doi: 10.1021/jm401680a. [DOI] [PubMed] [Google Scholar]
- 92.Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 93.Afacan NJ, Yeung ATY, Pena OM, Hancock REW. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr Pharm Des. 2012;18:807–19. doi: 10.2174/138161212799277617. [DOI] [PubMed] [Google Scholar]