Abstract
Background and Purpose
Efforts to reduce disparities in recurrent stroke among Black and Latino stroke survivors have met with limited success. We aimed to determine the effect of peer education on secondary stroke prevention amongst predominantly minority stroke survivors.
Methods
Between 2009 and 2012, we enrolled 600 stroke or transient ischemic attack survivors from diverse, low-income communities in New York City into a 2-arm randomized clinical trial that compared a 6 week (1 session/week) peer-led, community-based, stroke prevention self-management group workshop (N=301) to a wait-list control group (N=299). The primary outcome was the proportion with a composite of controlled blood pressure (<140/90 mmHg), low density lipoprotein (LDL) cholesterol < 100 mg/dL, and use of antithrombotic medications at 6 months. Secondary outcomes included control of the individual stroke risk factors. All analyses were by intent-to-treat.
Results
There was no difference in the proportion of intervention and control group participants’ achieving the composite outcome (34% versus 34%, p=0.98). The proportion with controlled blood pressure at 6 months was greater in the intervention group than in the control group (76% versus 67%; p=0.02). This corresponded to a greater change in systolic blood pressure in the intervention versus control group (−3.63 SD 19.81 mm Hg versus +0.34 SD 23.76 mmHg; p=0.04). There were no group differences in the control of cholesterol or use of antithrombotics.
Conclusions
A low-cost peer education self-management workshop modestly improved blood pressure, but not LDL cholesterol or antithrombotic use, among stroke and TIA survivors from vulnerable, predominantly minority urban communities.
Keywords: stroke prevention, disparities, self-management, randomized trial
Stroke is the fourth leading cause of mortality and a leading cause of disability in the United States (US).1 Over the next two decades, the proportion of the US population with a history of stroke is expected to rise to close to 4% and stroke-associated healthcare costs are expected to increase by 129%.2 There are also long-standing disparities in the incidence of stroke and TIA in minority populations in the US.3–7 Although stroke-related mortality has steadily declined in the US since the 1950s, stroke death rates have remained higher in blacks than in whites.1 Minority groups are also at greater risk for recurrent stroke. Two studies, including one in Northern Manhattan, found a two to three-fold increased risk for recurrent stroke among African Americans and Latinos relative to whites.8,9
A prior history of a stroke or transient ischemic attack (TIA) represents the strongest risk marker for future stroke.8, 10–12 Three of the most important actions stroke and TIA survivors can take to reduce their risk of future strokes include controlling blood pressure to a goal of less than 140/90 mmHg,13 controlling cholesterol to a goal low density lipoprotein (LDL) of less than 100 mg/dL,14, 15 and – unless the stroke represents a hemorrhagic event – taking an antithrombotic medication.16, 17 Yet, control of these stroke prevention measures among stroke and TIA survivors remains suboptimal, particularly among individuals from minority groups.18–22 Accordingly, differences in the control of these stroke risk factors likely explain, at least in part, disparities in prognosis in stroke and TIA survivors.
Given the increasing burden of stroke in the US and the heightened risk of stroke among stroke and TIA survivors from minority groups, there is a pressing need to develop interventions that improve stroke risk factors, particularly among individuals from historically disadvantaged groups such as Latinos and African Americans in the United States. Evidence suggests that self-management education is a promising approach to improving outcomes for individuals in these populations.23–25 Some of these programs employ peer educators or community health workers who are trusted and respected members of the community, culturally and linguistically compatible with the target population, and hence, well suited to facilitating social support, education, access, adherence, and promotion of self-care.26–28 The provision of peer education in a group-based format with stroke/TIA survivors from one’s community may be conducive to a supportive environment that enhances the effectiveness of peer education.
Accordingly, we employed a community-based participatory research approach29 to develop a culturally-tailored, peer-led stroke prevention group-based workshop adapted from the Chronic Disease Self Management Program.30 The primary goal was to determine whether participation in such a workshop could increase the proportion of stroke and TIA survivors who achieve a composite outcome of control of blood pressure (<140/90 mm Hg), lipids (LDL cholesterol <100 mg/dL), and regular use of anti-thrombotic medications. Secondary outcomes of interest included control of the individual stroke prevention measures, change in blood pressure and LDL cholesterol assessed as continuous variables, medication adherence, and depressive symptoms.
Methods
Study Population
Between June 2009 and January 2012, our community-academic team recruited individuals with a history of stroke or TIA through screenings at senior centers, churches, and health fairs; contacting patients with a history of a stroke on hospital registries of an academic medical center, a federally-funded community health center, and a home care nursing program; and advertising the study in clinics, community organizations, and newspapers based in the Upper Manhattan and South Bronx neighborhoods of New York City. Participants were eligible if they were at least 40 years of age and if they reported the occurrence of a stroke or “mini-stroke” (i.e., TIA) within the past 5 years. Participants were excluded if they did not have capacity to provide informed consent, if they did not have the physical or mental capacity to participate meaningfully in self-management workshops (e.g., severe aphasia or cognitive impairment), if they were non-English- and non-Spanish-speaking, or if they resided in an institutionalized setting. The local institutional review board approved the study, and all participants provided written informed consent. Additional details about the Prevent Recurrence of All Inner-City Strokes through Education (PRAISE) trial study methods have been published elsewhere.30
Study Design
The study was a 2-arm randomized controlled trial with a 6-month follow-up. After in-person enrollment and baseline assessment, participants returned for an in-person interview at 6-months to determine study outcomes.
Baseline Assessments
At baseline, research assistants confirmed each participant’s eligibility, assessed their demographic status, reviewed medication lists, surveyed medical problems to obtain a Charlson Comorbidity Index31, and assessed stroke-related disability using the Modified Rankin Scale.32 Trained study personnel also administered validated self-report measures of depressive symptoms and medication adherence, measured blood pressure, and collected blood to measure LDL cholesterol. Participants brought medication bottles to provide prescription information.
Randomization
After completion of baseline assessments, research assistants allocated participants to a randomly selected group. Randomization was generated by a computerized random-number sequence in blocks of two, four, or six people. Participant assignments were placed in sealed, opaque envelopes by a third party who had no contact with participants. Randomization was stratified by recruitment approach; those recruited from community-based, academic medical center, community health center, and the homecare nursing agency were randomized separately.
Treatment Groups
Peer Education Intervention Group
Participants randomized to the peer education intervention group were scheduled to attend a weekly peer-led workshop for six weeks with each session scheduled for ninety minutes. The workshop was modeled on the Chronic Disease Self-Management Program in which participants learn and practice self-management skills in a supportive group environment.25, 33 The workshop was comprised of didactic components that 1) explained the biology of stroke and stroke treatments in terms lay people could understand; 2) stressed the importance of adherence to preventive medications to reduce stroke recurrence; and 3) provided suggestions for optimizing medication adherence and working with a healthcare team. Sessions took place in groups of eight to ten participants in either English or Spanish and were led by two peer leaders with similar socioeconomic backgrounds and health problems as the participants. Participants could bring a family member, friend, or home attendant if they chose. At the end of each session, participants were asked to make an “action plan” which specified a concrete step they could take to help prevent a recurrent stroke. They were encouraged to choose something relevant to what they had learned during that week’s session. Subsequent sessions would begin with participants reporting back to the group on the results of their efforts to implement this action plan. Hence, key ingredients of the peer education intervention workshop included modeling of self-management and problem-solving techniques, guidance for self-management, weekly action planning for specific behaviors, feedback on progress, and social persuasion through group support. At the randomization visit, participants in the intervention group additionally received a small packet of culturally-sensitive stroke education materials and a list of local health providers, including those that accepted patients without health insurance. Participants also received their baseline results pertaining to their blood pressure and LDL cholesterol in writing and were encouraged to discuss these results with their health care provider.
Wait-List Control Group
Participants in the wait-list control group were told they would receive access to the peer education workshops at no cost after a one-year waiting period. At the randomization visit, they received the same small packet of stroke education materials, list of local health providers, and advice to discuss blood pressure and cholesterol results with their health care providers as participants in the intervention group.
Follow-Up Assessments
Six months after randomization, participants in both groups returned for in-person follow-up assessments. Data collection at the 6-month study visit was similar to the baseline visit. Research assistants who performed follow-up assessments were blinded to group assignment.
Outcomes and Measurements
The primary outcome was the proportion of participants who achieved goals for all three key stroke prevention measures - blood pressure (<140/90 mmHg), LDL cholesterol <100 mg/dL, and antithrombotic use at 6 months. Key secondary outcomes included the proportion with control of blood pressure, LDL cholesterol, and antithrombotic use, as individual prevention measures, as well as change in within-participant systolic and diastolic blood pressure, and change in within-participant LDL cholesterol; change in the proportion with good self-reported medication adherence between baseline and 6 months; and change in the proportion with elevated depressive symptoms between baseline and 6 months.
Trained research assistants measured blood pressure using a BP Tru automatic sphygomanometer according to the American Heart Association guidelines.34 The average of the last two of three readings was used for analyses. LDL cholesterol was measured using the direct method, eliminating the need for fasting.35 Use of anti-thrombotic medication was assessed by a review of medication bottles during the baseline visit or by asking patients to read the names of all their pill bottles at home by telephone. Consistent with the recent recommendations of the American Heart Association/American Stroke Association guidelines16, participants were considered to be using an appropriate antithrombotic medication if they took aspirin (greater than 50mg per day at least three days per week), clopidogrel, or the combination of aspirin and dipyridamole as antiplatelet therapy, or anticoagulation with warfarin or equivalent for patients with atrial fibrillation and had no contraindication to antiplatelet and anticoagulant medication. We measured medication adherence using the Morisky Medication Adherence Scale,36 which includes eight items that assess pill-taking behaviors. Summary scores on the scale can classify individuals into non-adherent (<6 points) and adherent (6 to 8 points) groups and these categorizations are concordant with a pharmacy refill measure of medication adherence.37 The team measured depressive symptoms using the 8-item version of the Patient Health Questionnaire,38 a well-validated measure of depressive symptoms in racially and ethnically diverse patients39 which has been validated in stroke survivors.40 A score of 10 or higher has a sensitivity of 91% and specificity of 89% for diagnosing depression in stroke survivors. The 8-item version is identical to the 9-item version except it omits one item asking about suicidal ideations.41
Statistical Analysis
We evaluated differences between participants randomized to the intervention and control groups using t-tests for continuous variables and chi-square analysis for categorical variables. We used intent-to-treat analyses to compare participants on all key outcomes. As a sensitivity analysis, we used generalized estimating equations (GEE) to compare the likelihood of achieving the composite outcome and the individual stroke prevention measures at 6-months in the intervention group as compared to the control group after accounting for control status at baseline, We used mixed-models to compare the change in blood pressure and change in lipids between baseline and 6 months between patients in the intervention and control groups. Missing values were imputed using multiple imputation under the assumption that values were missing at random. As our findings were similar when we performed analyses with and without imputing missing values, we only present data pertaining to our imputed data. We additionally performed exploratory, (i.e., not pre-specified) subgroup analyses for the effect of the intervention on blood pressure by sex and race/ethnicity, and tested for significance using a treatment*subgroup interaction test.
Sample size was calculated for the composite outcome of combined control of blood pressure, LDL cholesterol, and antithrombotic use. Based on prior literature,20 we used a conservative estimate of baseline use of stroke prevention measures (40%) for our sample size estimate. With 80% power, 5% significance level, and a two-tailed test, we estimated we would need 270 participants per group to detect a 20% relative difference in the proportion of participants in the intervention group who achieved the composite outcome as compared to the proportion achieving the composite outcome in the control group (i.e., 70% in intervention arm versus 56% in control arm). To account for loss to follow-up, we increased the sample size to 600. All analyses were conducted based on the intention-to-treat principle. PASS (version 08.0.13, NCSS, LLC, Keysville, UT) was used for sample size calculation and SAS 9.2 software (SAS Institute Inc., Cary, NC) was used for all other analyses.
Results
Of 2,665 individuals who were screened for study participation, 52% were ineligible, 24% declined, and 600 enrolled and were randomized (301 intervention; 299 control) (Figure). We retained 85% at 6 month follow up. There were no significant differences in key sociodemographic or clinical characteristics between participants who did and did not complete follow-up.
Figure.
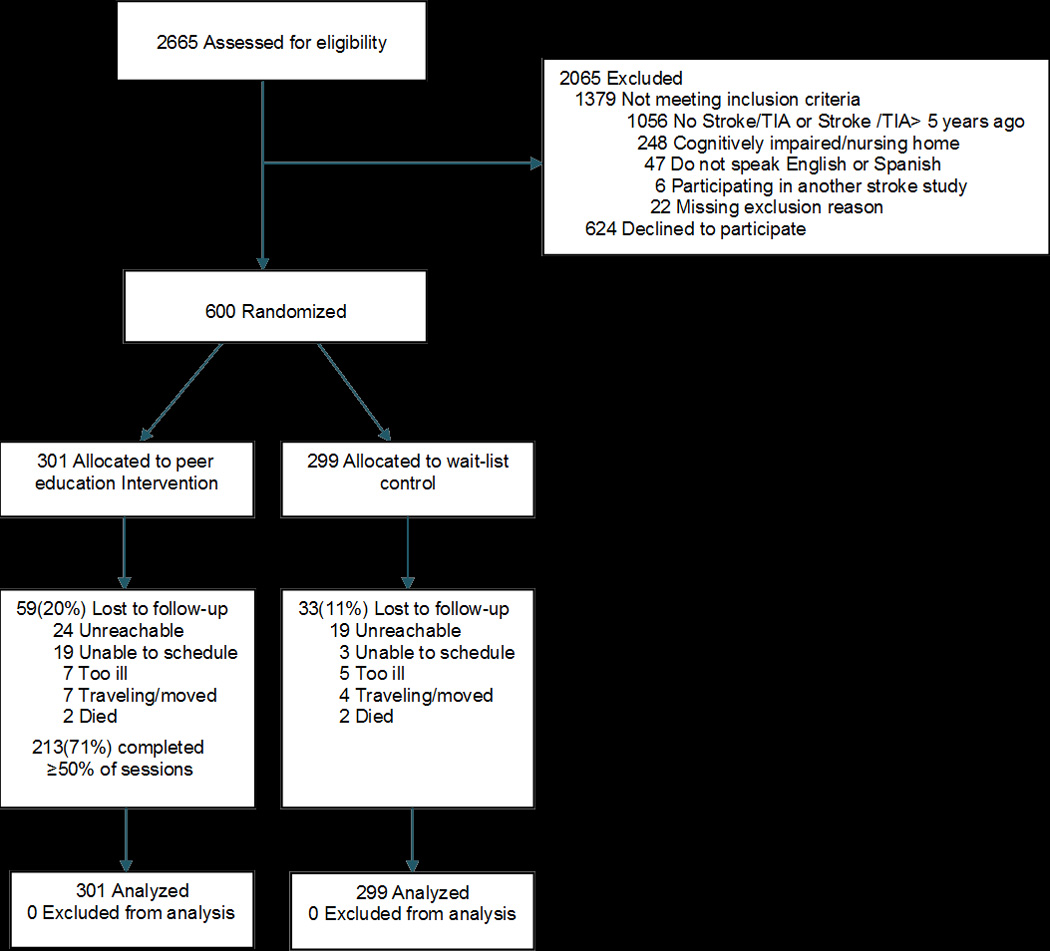
CONSORT (Consolidated Standards of Reporting Trials) Diagram of the Prevent Recurrence of All Inner-City Strokes through Education (PRAISE) trial
Abbreviations: TIA, transient ischemic attack
Participants randomized to the intervention and control groups were similar in demographic and clinical variables at baseline (Table 1). The mean (SD) age of participants was 63 (11) years, 59% were women, 86% were non-white, 57% had an annual household income of less than $15,000, and nearly one-third had a less than high school education. Approximately one-third had blood pressure above goal, 42% had LDL cholesterol above goal, 18% were not taking an antithrombotic medication, and only 35% were at goal for all three stroke prevention measures. Forty percent reported nonadherence to medications. Among participants randomized to the intervention, the mean number of workshop sessions attended was four. Seventy-one percent attended at least 50% of the scheduled sessions, and 16% did not attend any sessions.
Table 1.
Baseline Patient Characteristics
| Patient Characteristics | Peer Education Intervention Group (N=301) |
Wait List Control Group (N=299) |
P Value |
|---|---|---|---|
| Demographic | |||
| Age, mean (SD), y | 63 ± 11 | 64 (11) | 0.59 |
| Female, % | 60 | 59 | 0.82 |
| Race or ethnicity, % | 0.39 | ||
| Black | 40 | 43 | |
| Latino | 42 | 37 | |
| White | 13 | 14 | |
| Other | 4 | 6 | |
| Annual income ≤ $15,000, % | 56 | 58 | 0.62 |
| Less than high school education, % | 31 | 30 | 0.98 |
| Insurance | 0.97 | ||
| Medicaid, % | 29 | 30 | |
| Medicare, % | 39 | 40 | |
| Commercial, % | 26 | 24 | |
| Uninsured % | 6 | 6 | |
| Clinical | |||
| Years since last stroke or TIA, mean (SD) | 1.8 (1.4) | 1.8 (1.5) | 0.79 |
| Systolic BP, mean (SD), mm Hg | 131 (22) | 132 (22) | 0.59 |
| Diastolic BP, mean (SD), mm Hg | 78 (12) | 78 (12) | 0.46 |
| Use antihypertensive medication, % | 78 | 83 | 0.11 |
| LDL cholesterol, mean (SD) | 97 (38) | 97 (35) | 0.80 |
| Use of anti-lipid medication, % | 65 | 63 | 0.63 |
| Charlson Co-morbidity Index, mean ± SD | 3.7 (2.1) | 3.6 (2.1) | 0.44 |
| Modified Rankin Score 3 or 4, % | 48 | 46 | 0.59 |
| Depressed (PHQ score ≥10), % | 31 | 28 | 0.39 |
| Nonadherent to medications, % | 42 | 38 | 0.41 |
Abbreviations: BP, blood pressure; LDL, low-density lipoprotein; TIA, transient ischemic attack; PHQ, Patient Health Questionnaire-8 item version
There was no difference in the proportion of intervention and control participants who at 6 months had attained all three stroke prevention measures (34% versus 34%, p=0.98). Furthermore, there were no differences in the proportion of intervention and control participants who at 6 months had controlled LDL cholesterol (54% versus 58%, p=0.46) or who took an anti-thrombotic medication (82% versus 84%, p=0.61). A higher proportion of participants randomized to the intervention had controlled blood pressure at 6 months as compared to control participants (76% versus 67%; p=0.02; Table 2). This corresponded to 1.13 times (95% CI: 1.02–1.25) increased likelihood of having controlled blood pressure at 6 months if assigned to the intervention compared to the control group. In the sensitivity analysis that controlled for baseline blood pressure control status, the difference in blood pressure control status at 6 months attributable to the intervention was nearly statistically significant (p=0.07). Participants in the intervention group had a greater change in systolic blood pressure at 6 months than control participants (−3.63 SD 19.81 mm Hg versus +0.34 SD 23.76 mmHg, p=0.04), but no significant difference in diastolic blood pressure (−1.95 SD 10.26 mm Hg versus −0.63 SD 11.88 mm Hg; p=0.18). The proportion of participants who were adherent increased by 7.4% in the intervention group compared with 1.0% in the control group (p=0.16). The proportion who were depressed declined by 10.5% in the intervention group compared with 5.7% in the control group (p=0.16). There were no significant interactions between the effect of the intervention and either sex or race/ethnicity.
Table 2.
Control of Stroke Prevention Measures at Baseline and 6 Months
| Variable | Intervention Group (N=299) |
Control Group (N=301) |
Relative Risk (95%CI) |
P- Value |
|---|---|---|---|---|
| Control of 3 stroke prevention measures at 0 mo, % | 37 | 33 | 1.09 (0.88 – 1.36) | 0.43 |
| Control of 3 stroke prevention measures at 6 mo, % | 34 | 34 | 1.00 (0.80 – 1.25) | 0.98 |
| Change in % with control of 3 stroke prevention measures | n/a | 0.51* | ||
| Controlled BP at 0 mo, % | 68 | 66 | 1.03 (0.92 – 1.16) | 0.60 |
| Controlled BP at 6 mo, % | 76 | 67 | 1.13 (1.02 – 1.25) | 0.02 |
| Change in % with controlled BP | n/a | 0.07* | ||
| Controlled LDL cholesterol at 0 mo, % | 58 | 58 | 1.02 (0.88 – 1.16) | 0.81 |
| Controlled LDL cholesterol at 6 mo, % | 54 | 58 | 0.95 (0.82 – 1.10) | 0.46 |
| Change in % with controlled LDL cholesterol | n/a | 0.30* | ||
| Taking anti-thrombotic at 0 mo, % | 81 | 84 | 0.96 (0.89 – 1.04) | 0.30 |
| Taking anti-thrombotic at 6 mo, % | 82 | 84 | 0.98 (0.91 – 1.06) | 0.61 |
| Change in % taking anti-thrombotic medication | n/a | 0.79* |
Abbreviations: BP, blood pressure; CI, confidence interval; LDL, low-density lipoprotein
Generalized estimating equations were used to estimate the likelihood of achieving controlled stroke prevention measures at 6-months in the intervention group as compared to the control group, after adjusting for control status at baseline. Significance tests are tests of the group × time interaction.
Discussion
In this study, participants randomized to a peer led community-based stroke education workshop as compared to a wait-list control group did not achieve greater combined control of three key stroke prevention measures, nor did they improve their control of LDL cholesterol or antithrombotic use when these stroke prevention measures were examined individually. A greater proportion of stroke and TIA survivors randomized to the intervention did, however, achieve controlled blood pressure, and this was associated with a group difference in systolic blood pressure of approximately 4 mm Hg. The intervention was also associated with a trend toward an improvement in medication adherence and depression.
Controlling blood pressure remains the cornerstone of the prevention of recurrent stroke.17, 42 A meta-analysis of the effects of antihypertensive medications showed that a reduction in systolic blood pressure of 5 mm Hg was associated with a 20% reduction in stroke.43 Although the optimal target blood pressure for stroke survivors is still being explored, reductions in blood pressure have been associated with lower rates of stroke even among stroke survivors with controlled blood pressure.43, 44 To put our intervention in perspective, a recent Cochrane meta-analysis of interventions to improve modifiable risk factors in stroke survivors found that there were no significant benefits of organizational, educational, or behavioral interventions on risk factor control.45 Accordingly, this peer led approach to improving stroke outcomes may be welcomed as a means of improving stroke outcomes, particularly in vulnerable communities with a high proportion of low-income minorities.
In contrast, with blood pressure control, achieving LDL cholesterol goals below 100 mg/dL was a class B recommendation according to the American Stroke Association guidelines at the time the study was conducted,17 and the newest guidelines for managing cholesterol suggest that use of statins might be a more important target for stroke prevention than LDL number in future studies.46 As LDL cholesterol is largely dependent on prescribing practices of clinicians, clinical uncertainty about the importance of LDL cholesterol goals may have limited the impact of this patient intervention on this goal. With respect to antithrombotic use, over 80% of participants in both groups were taking antithrombotics at baseline. As participants were, in part, enrolled from non-medical settings, we did not have access to medical records, and could not ascertain the type of stroke (hemorrhagic versus ischemic) nor the proportion with an indication for antithrombotic medication for stroke prophylaxis. In population-based samples, about 10% of stroke survivors have contraindications to antithrombotics. Hence, we may have been unable to demonstrate an effect of our intervention on this stroke prevention measure due to ceiling effects.
There were several notable strengths of this study. In partnership with our community action board, we successfully recruited a group of stroke and TIA survivors who are often excluded from clinical trials.47 Many of our participants were non-white, had incomes below the poverty level, lacked health insurance, and had significant levels of disability and distress as measured by the modified Rankin score and the PHQ, respectively. Despite these vulnerabilities, the majority of participants attended more than half of the workshop sessions, confirming that such an approach is feasible, even among low-income, minority stroke survivors. This community-based, peer led intervention has the potential to be more easily sustained in the low-income, non-white communities at highest risk for stroke as opposed to other more resource-intensive strategies such as individualized case-management or tailored health education that involve significant time and effort by health care professionals.45
The study also had several limitations. The generalizability of our findings to other stroke and TIA survivor populations remains unknown. Recruitment required substantial effort and led to the inclusion of many participants already reaching study goals for stroke prevention. The optimal approach to identifying participants for such an intervention would benefit from careful consideration. For example, limiting recruitment to stroke centers or TIA clinics may facilitate a more targeted approach but may exclude the large number of stroke/TIA survivors who do not follow at such centers. We did not utilize an attention control group, and thus, it is difficult to determine which aspects of the intervention were most important for achieving the improvement in blood pressure control. Future studies could assess the extent to which the use of peer educators is a key component of chronic disease self-management interventions, particularly in minority populations. The provision of an educational packet to participants in the wait-list control group may have contributed to a contamination bias that would have biased our results to the null. We did not assess the extent to which blinding was maintained among research assistants performing outcomes assessments. Our measure of medication adherence was based on self-report and was not specific to stroke medications. Future studies could examine the impact of the intervention on objective measures of adherence to stroke prevention medications to determine the extent to which such approaches lead to better risk factor control through increased medication or lifestyle adherence or through empowering patients to be proactive when visiting their clinicians.
In summary, a peer education workshop can be successfully delivered to stroke and TIA survivors in vulnerable communities and can produce modest improvements in blood pressure control. Given the strong association between blood pressure and stroke risk, this peer led approach may be helpful for reducing disparities in stroke outcomes. Future studies should assess the effect of targeting this type of intervention to stroke/TIA survivors who are not reaching goals for stroke prevention.
Acknowledgements
We gratefully acknowledge the East and Central Harlem Community Action Board and the study participants for their generous contributions.
Sources of Funding
Dr. Kronish received support from the National Heart, Lung and Blood Institute (K23 HL098359) and the National Center for Advancing Translational Science (UL1TR000040). Dr. Horowitz, Dr. Goldfinger, Dr. Tuhrim, Ms. Negron, Ms. Arniella, and Ms. Fei received support from the National Institute of Minority Health and Health Disparities (P60MD00270) and Dr. Horowitz received funding from the National Center for Research Resources (UL1RR029887).
Footnotes
Conflicts of Interest
None
Financial Disclosures
None
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the united states: A policy statement from the american heart association and american stroke association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 3.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: The northern manhattan study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 4.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in mexican americans compared with non-hispanic whites: The brain attack surveillance in corpus christi project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 7.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: Epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 8.Sacco RL, Shi T, Zamanillo MC, Kargman DE. Predictors of mortality and recurrence after hospitalized cerebral infarction in an urban community: The northern manhattan stroke study. Neurology. 1994;44:626–634. doi: 10.1212/wnl.44.4.626. [DOI] [PubMed] [Google Scholar]
- 9.Sheinart KF, Tuhrim S, Horowitz DR, Weinberger J, Goldman M, Godbold JH. Stroke recurrence is more frequent in blacks and hispanics. Neuroepidemiology. 1998;17:188–198. doi: 10.1159/000026172. [DOI] [PubMed] [Google Scholar]
- 10.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes : A population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 11.Hankey GJ. Impact of treatment of people with transient ischaemic attacks on stroke incidence and public health. Cerebrovasc Dis. 1996;6:26–32. [Google Scholar]
- 12.Clark TG, Murphy MF, Rothwell PM. Long term risks of stroke, myocardial infarction, and vascular death in "low risk" patients with a non-recent transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2003;74:577–580. doi: 10.1136/jnnp.74.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 15.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the american heart association/american stroke association council on stroke. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 17.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 18.Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: The reasons for geographic and racial differences in stroke study. Stroke. 2006;37:1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 19.Hillen T, Dundas R, Lawrence E, Stewart JA, Rudd AG, Wolfe CD. Antithrombotic and antihypertensive management 3 months after ischemic stroke : A prospective study in an inner city population. Stroke. 2000;31:469–475. doi: 10.1161/01.str.31.2.469. [DOI] [PubMed] [Google Scholar]
- 20.Tuhrim S, Cooperman A, Rojas M, Brust JC, Koppel B, Martin K, et al. The association of race and sex with the underuse of stroke prevention measures. J Stroke Cerebrovasc Dis. 2008;17:226–234. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Ruland S, Raman R, Chaturvedi S, Leurgans S, Gorelick PB. Awareness, treatment, and control of vascular risk factors in african americans with stroke. Neurology. 2003;60:64–68. doi: 10.1212/wnl.60.1.64. [DOI] [PubMed] [Google Scholar]
- 22.Sanossian N, Wu J, Azen SP, Varma R. Prevalence and risk factors for cerebrovascular disease in community-dwelling latinos. Clin Neurol Neurosurg. 2008;110:985–987. doi: 10.1016/j.clineuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Meta-analysis: Chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 24.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478–488. [PMC free article] [PubMed] [Google Scholar]
- 25.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Brownstein JN, Bone LR, Dennison CR, Hill MN, Kim MT, Levine DM. Community health workers as interventionists in the prevention and control of heart disease and stroke. Am J Prev Med. 2005;29:128–133. doi: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Gary TL, Bone LR, Hill MN, Levine DM, McGuire M, Saudek C, et al. Randomized controlled trial of the effects of nurse case manager and community health worker interventions on risk factors for diabetes-related complications in urban african americans. Prev Med. 2003;37:23–32. doi: 10.1016/s0091-7435(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 28.Klein LA, Ritchie JE, Nathan S, Wutzke S. An explanatory model of peer education within a complex medicines information exchange setting. Soc Sci Med. 2014;111:101–109. doi: 10.1016/j.socscimed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 29.James SAG, Bickell NA, Walker W, Robinson V, Taylor B, Horowitz CR. Community action boards: An innovative model for effective community-academic research partnerships. Progress in Community Health Partnerships: Research, Education, and Action. 2011;5:399–401. [PMC free article] [PubMed] [Google Scholar]
- 30.Goldfinger JZ, Kronish IM, Fei K, Graciani A, Rosenfeld P, Lorig K, et al. Peer education for secondary stroke prevention in inner-city minorities: Design and methods of the prevent recurrence of all inner-city strokes through education randomized controlled trial. Contemp Clin Trials. 2012;33:1065–1073. doi: 10.1016/j.cct.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 33.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 35.Dungan KM, Guster T, DeWalt DA, Buse JB. A comparison of lipid and lipoprotein measurements in the fasting and nonfasting states in patients with type 2 diabetes. Curr Med Res Opin. 2007;23:2689–2695. doi: 10.1185/030079907x233304. [DOI] [PubMed] [Google Scholar]
- 36.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the patient health questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams LS, Brizendine EJ, Plue L, Bakas T, Tu W, Hendrie H, et al. Performance of the phq-9 as a screening tool for depression after stroke. Stroke. 2005;36:635–638. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: A systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 43.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boan AD, Lackland DT, Ovbiagele B. Lowering of blood pressure for recurrent stroke prevention. Stroke. 2014;45:2506–2513. doi: 10.1161/STROKEAHA.114.003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lager KE, Mistri AK, Khunti K, Haunton VJ, Sett AK, Wilson AD. Interventions for improving modifiable risk factor control in the secondary prevention of stroke. Cochrane Database Syst Rev. 2014;5:CD009103. doi: 10.1002/14651858.CD009103.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 47.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: The northern manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]


