Abstract
Objective
Extent of vocal fold injury impacts the nature and timing of wound healing, and voice outcomes. However, depth and extent of the lesion created to study wound healing in animal models vary across studies, likely contributing to different outcomes. Our goal was to create a surgery classification system to enable comparison of postoperative outcomes across animal vocal fold wound healing studies.
Study design
Prospective, controlled animal study.
Methods
Rats underwent one of three types of unilateral vocal fold surgeries classified by depth and length of resection. The surgeries were a subepithelial injury, resection of epithelium and superficial layer of the lamina propria at the midmembranous portion of the vocal fold; transmucosal injury, resection of epithelium and lamina propria; and transmuscular injury, resection of epithelium, lamina propria and superficial portion of the vocalis muscle Wound healing was evaluated histologically at various time points up to 35 days post-injury.
Results
Complete healing occurred by 14 days post-surgery for subepithelial injury and by day 35 for transmucosal injury. Injury remained present at day 35 for transmuscular injury.
Conclusions
Timing and completeness of healing varied by extent and depth of resection. Scarless healing occurred rapidly following subepithelial injury, while scarring was observed at five weeks after transmuscular injury. The proposed classification system may facilitate comparison of surgical outcomes across vocal fold wound healing studies.
Keywords: surgical classification, vocal fold injury, wound healing, animal model
INTRODUCTION
Phonomicrosurgery is performed to conserve or improve vocal function. However, an important risk associated with phonomicrosurgery is worsened voice quality resulting from postoperative scarring. In vivo animal models have been used widely to study vocal fold scarring to elucidate the mechanism of vocal fold repair and to prevent scarring or to develop restorative treatments. Studies on rats1,2, rabbits3,4, dogs5,6, pigs7,8,9, and mice10,11 have yielded important insights into the sequence and timing of wound healing events in the vocal fold epithelium, lamina propria, and muscle. However, differences in surgical techniques used, as well in the type and extent of damage to the vocal fold cover and body created, likely impact the nature and timing of the observed wound healing process. In the present study, we created a classification system to categorize vocal fold injury according to the extent and depth of injury. Furthermore, we sought to examine the effects of injury type on timing and completeness of healing. Finally, we sought to relate the proposed classification system to vocal fold surgeries performed in clinical settings.
The scope of vocal fold surgeries in clinical practice can range from removal of epithelium for biopsy to resection of the cover and body. For some patients, vocal fold outcomes are suboptimal. It has been hypothesized that differences in outcomes may be attributable to the extent of injury as well as patient-specific characteristics12. Because of practical and ethical constraints in accessing human tissue, the histologic features of scarred human vocal fold have been reported rarely12. Therefore, animal models have proved indispensable for allowing systematic study of the nature and mechanisms of wound healing. Here, we elected to use a rat model to develop a classification system of injury. We acknowledge that larger animals, including rabbits13 pigs7–9,14,15 and dogs16 have several advantages over the rat. For example, like rats, large animals demonstrate morphological similarities to the human larynx and can be used to study the vibratory property of vocal folds post-injury17; unlike rats, the large size of their larynx permits relatively facile study of rheological properties of the vocal folds following injury. This confers large animal models with an important advantage over rats as it permits insights into the likely effects of extent and depth of injury on phonation. While recognizing the limitations of the rat model, we chose to study injury in rats as they offered the least sentient animal that met criteria for use in this line of research. First, the rat and human vocal fold share structural similarities1; for example, rats and humans share a trilayered vocal fold the morphology and fibrous protein composition of Reinke’s space is comparable to that of humans18. Second, as the rat model has been used extensively to study wound healing in vocal fold mucosa; it is the model for which there is the most data available on structural, genetic and molecular changes in the mucosa following injury19,20. Third, the size of the rat larynx also allows ex vivo vibratory analyses. Finally, the rat model is cost-efficient relative to larger animal models.
While surgical injury to the rat is commonly used to explore wound healing, the type of injury created differs across studies1,2,17,19, 21–28. In all the papers reviewed, investigators used endoscopy-guided procedures to create an injury. The type of instrumentation used and the description of the margins of injury varied across studies. In terms of instrumentation, investigators used microscissors, microforceps, and/or needles ranging in gauge from 22 to 27 to create injuries (Supplementary Table 1). Occasionally manuscripts did not provide sufficient details regarding surgical methods in order to draw comparisons regarding methodologies used. Surgical methods chosen likely influenced healing outcomes, consequently rendering a comparison of findings across studies difficult. A classification system could facilitate standardization of injuries and comparison of outcomes across studies.
Clinically, classification for vocal fold surgery to remove cancerous lesions has been advocated to compare postoperative results or minimize the removal extension and depth. The European Laryngological Society proposed a classification system for endoscopic cordectomies according to the degree of resection performed29,30. That classification system proposed eight types of surgery, described according to the margins of surgery. Guided by that work, we hypothesized that a surgical classification for rat vocal fold injury model could be created and would facilitate comparison of findings across rat studies. We further hypothesized that degree of injury would influence histopathological outcomes. We proposed three types of injury which include a subepithelial injury, which is a resection of the epithelium and superficial layer of the lamina propria; transmucosal injury, which is a resection of the epithelium and all layers of the lamina propria, without damaging the muscle; and transmuscular injury, which proceeds through part of the vocalis muscle (Figure 1). To assess and compare the post-operative results of different types of rat vocal fold injury, we compared the effect of injury type of the timing and extent of healing. Based on the phonosurgery classification system outlined by the European Laryngological Society31 (Friedrich et al., 2007), we propose that a subepithelial injury would be performed clinically to remove papillomatosis, hyperplasia or carcinoma in situ. A transmucosal injury is would be used to resect nodules, polyps, cysts or sulci. Transmuscular injury would be consistent with clinical resection of carcinoma.
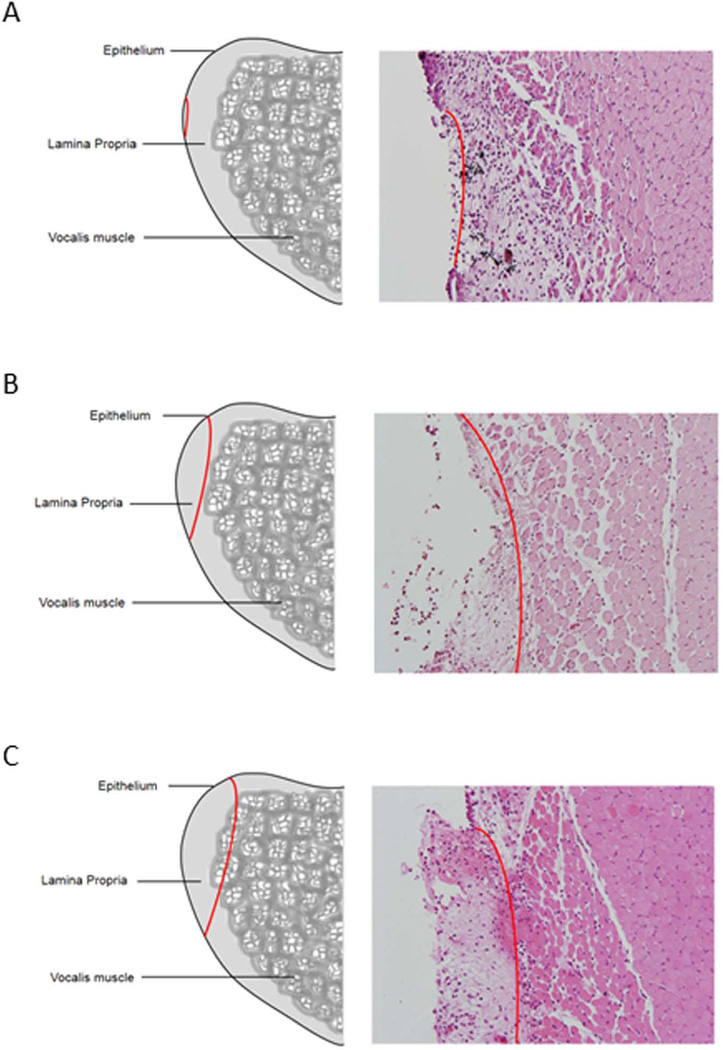
Figure 1. Classification system for rat injury.
Sketch and hematoxylin and eosin staining of three proposed injury types. The red line indicates the depth and extent of injury which ranges from A. resection of the epithelium and superficial layer of the lamina propria; transmucosal injury, B. resection of the epithelium and all layers of the lamina propria, and C. resection of the vocal fold mucosa and the superficial aspect of the vocalis muscle.
MATERIALS AND METHODS
Surgical procedure
Forty-fourtwo- to four-month-old male Sprague-Dawley rats were used in this study. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Wisconsin–Madison. Rats were anesthetized by exposure to isofluorane (2% to 3% delivered at 0.8–1.5 L/minute) and intraperitoneal injection of 90 mg/kg of ketamine hydrochloride and 9 mg/kg of xylazine hydrochloride. Intraperitoneal atropine sulfate was used to reduce salivation (0.05mg /kg) to facilitate visualization of the vocal folds. Animals were placed on a custom made operating platform in a near vertical position with the mouse secured open (Supplementary Figure 1A)32. Vocal folds were visualized using a custom-fabricated 1mm diameter steel wire laryngoscope and a 1.9mm diameter 30 degree endoscope (Karl Storz Endovision, Inc., Charlton, MA) connected to an external light source and video monitor (Supplementary Figure 1B). Injury was created with a 25-gauge needle (Supplementary Figure 1C). Each surgical procedure was performed by a surgeon with 10 years of experience performing human laryngeal surgeries and four years of experience conducting rat laryngeal surgeries performed. Appropriate depth and extent of resection was confirmed histologically for each surgery type at day 1 post-injury.
Three types of injuries were performed: subepithelial, transmucosal and transmuscular injuries. Healing over time was examined by harvesting three rats at each time point for each injury type. For subepithelial injury the right vocal fold was incised at medial surface along the membranous portion of the vocal fold and the t epithelial layer and superficial layer of the lamina propria were removed (Figure 1A). For transmucosal injury an incision was created on right vocal fold surface along the membranous portion of the vocal fold and the entire mucosal layer from the anterior commissure to the vocal process of the arytenoid was removed until the thyroarytenoid muscle was exposed (Figure 1B). For transmuscular injury, the right vocal fold was incised at medial surface and the mucosal layer was removed along the length of the vocal fold from the anterior commissure to the vocal process of the arytenoid. The fascia of vocalis muscle was incised and the superficial layer of muscle was removed along the muscle fibers (Figure 1C).
Rat larynges were harvested at days 1, 3, 5 and 14 post-injury for subepithelial injury and days 1, 3, 5, 14 and 35 for transmucosal and transmuscular injury. The rats were euthanized by an excess of carbon dioxide inhalation and all larynges were excised for histological evaluation (Figure 2D; online-only content). Two of the 44 rats (<5%) died due to complications related to anesthesia.
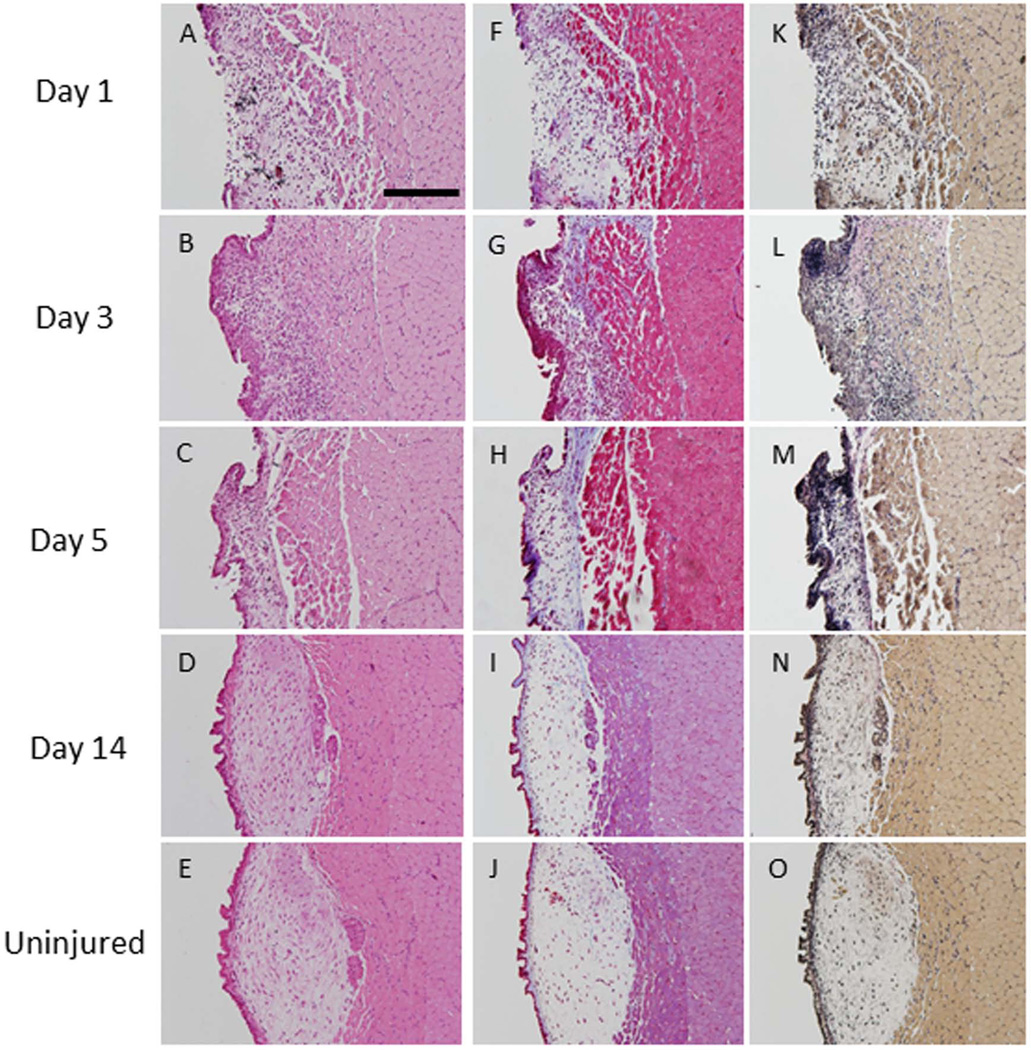
Figure 2. Subepithelial injury.
Hematoxylin and eosin staining at three time points post-injury (A–D) and in an uninjured vocal fold (E). Trichrome staining shows increased collagen density (blue) up to 5 days post-injury (F–H). Collagen deposition at day 14 (I) were indistinguishable from levels observed in uninjured tissue (J). Verhoff–van Gieson (VVG) staining showed increased elastin deposition (black) in the injured lamina propria up to 5 days post-injury (K-M) compared to uninjured vocal folds (O). At day 14 (N), elastin deposition was at uninjured levels (O) (200µm scale bar; original magnification 20×).
Histological analyses
Rat larynges were fixed in 10% neutral phosphate-buffered formalin overnight at room temperature and embedded in paraffin. Serial coronal sections of vocal folds were made 5µm along the entire length of the vocal folds from the anterior commissure to the vocal process of the arytenoids. Sections were prepared for routine hematoxylin and eosin (H&E), Masson trichrome, and Verhoff-van Gieson (VVG) staining. H&E staining was used for morphological analyses. Trichrome staining was used to evaluate collagen content. Elastin content was visualized with VVG staining. Both stains were analyzed qualitatively for density of labeling relative to uninjured control tissue by two raters. The raters were blinded to the purpose of the study. They achieved 93% agreement. Disagreement was resolved by consensus. All sections were viewed using a Nikon E600 microscope (Nikon, Melville, NY) and were photographed using an Olympus DP71 microscope digital camera (Tokyo, Japan).
RESULTS
Subepithelial injury
Epithelium was absent in the injured region at day 1 post-injury (Figure 2A). By day 3, complete reepithelialization was observed and inflammatory cells were present in the lamina propria (Figure 2B). No damage to the muscle was noted at any time point. Collagen deposition in the lamina propria, as evidenced by trichrome (blue) staining, was slightly elevated at day 1, peaked at day 3, and remained elevated through day 5 of injury (Figure 2F, G, H). By day 14, collagen deposition levels returned to baseline levels (Figure 2I, J). VVG staining confirmed these findings (blue staining). Further, VVG staining demonstrated an increase in elastin density (black staining) in the injured lamina propria at days 1, 3 and 5 post-injury (Figure 2K, L, M). By day 14, elastin levels of the injured vocal folds were indistinguishable from those of control vocal folds (Figure 2N, O). As injury was considered complete by 14 days post-injury, we did not harvest animals at a later time point. Findings were consistent across all rats.
Transmucosal injury
In all rats, the epithelial layer was absent and inflammatory cells were observed in the thin lamina propria at day 1. The muscle was not damaged (Figure 3A). At day 3, the epithelium regenerated but some inflammatory cells remained in the lamina propria at day 5 (Figure 3B). At days 5 and 14 post-injury, the epithelium was thickened and the volume of the lamina propria was reduced (Figure 3C, D) relative to uninjured tissue. By day 35, the injured vocal fold more closely resembled the uninjured vocal fold (Figure 3E, F).
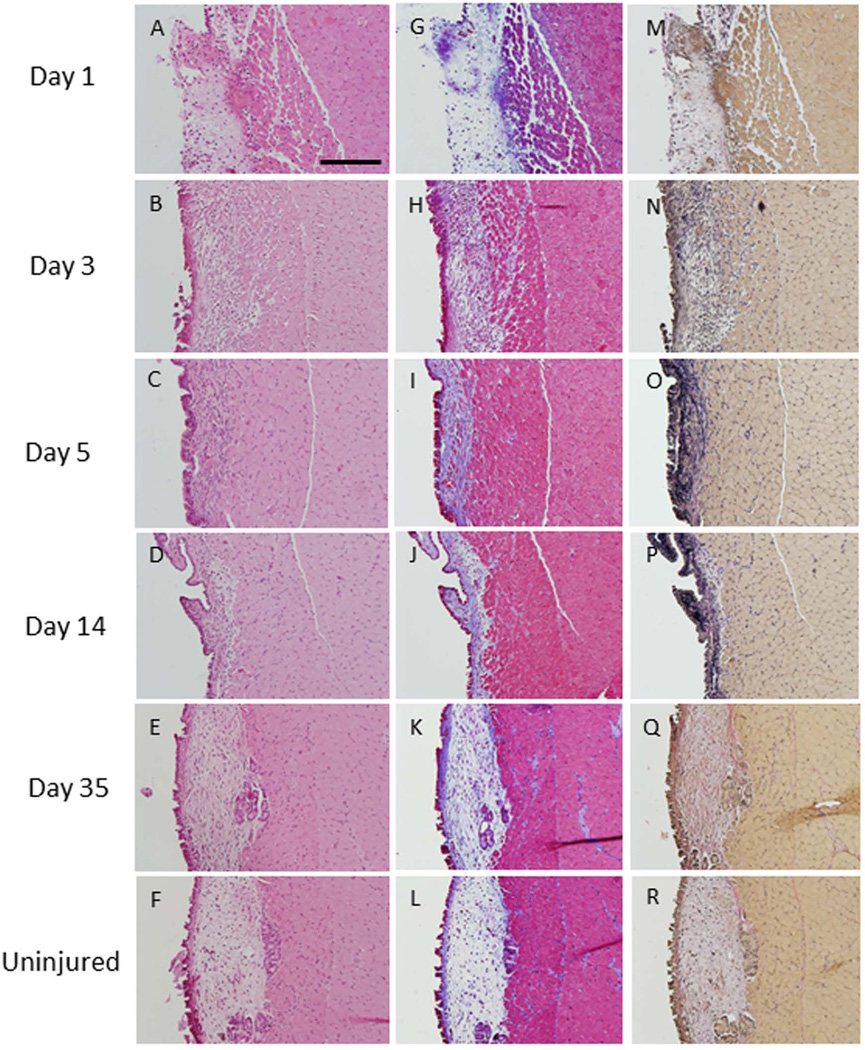
Figure 3. Transmucosal injury.
At day 1, epithelium was absent and inflammatory cells were observed in the lamina propria (A). The epithelium regenerated but inflammatory cells remained in the lamina propria at day 3 (B). At days 5 and 14, epithelial hyperplasia was noted and the volume of the lamina propria was reduced (C, D). The injured vocal fold closely resembled the non-injured vocal fold by day 35 (E, F). Trichrome staining showed elevated collagen deposition (blue s) at days 3, 5 and 14 (H–J). Collagen returned to baseline levels by day 35 (K, L). Elastin deposition (black) was elevated at days 3, 5 and 14 (N–P). Elastin returned to non-injured levels by day 35 (Q, R) (200 µm scale bar; original magnification 20×).
Collagen and elastin staining in the lamina propria were elevated during the first two weeks of wound healing. While no increase in collagen deposition was observed at day 1 (Figure 3G), collagen density was increased relative to non-injured tissue at day 3, 5 and 14 post-injury (Figure 3H, I, J). At day 35, collagen levels returned to non-injured levels (Figure 3K, L). Similarly, elastin levels were elevated at 3, 5 and 14 days post-injury (Figure 3N, O, P). At day 35, elastin returned to pre-injury levels (Figure 3M, Q, R).
Transmuscular injury
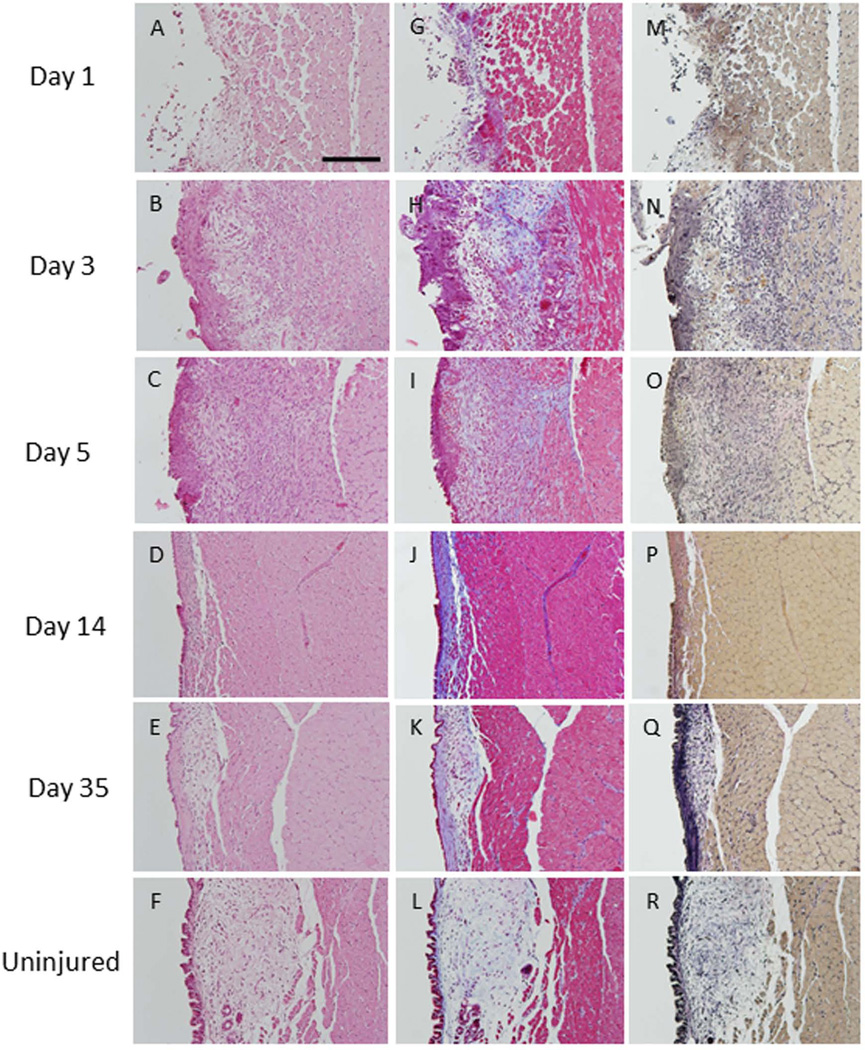
At day 1, the epithelium and most of the lamina propria were absent in all rats. Damage to the thyroarytenoid muscle was evidenced by disorganization of muscle fiber in the superficial layer of the muscle (Figure 4A). At day 3, epithelial hyperproliferation was present. By day 14, epithelial thickness returned to control levels. Many inflammatory cells were observed in the lamina propria at days 3 and 5 (Figure 4B). By day 5, granulation tissue was observed in the lamina propria (Figure 4C). At days 14 and 35, a reduction in lamina propria volume was observed (Figure 4D, E). Collagen density was elevated at day 3, 5, 14 and 35 post-injury (Figure 4H, I, J, K). Similarly, elastin density was elevated at day 3, and remained elevated through day 35 (Figure 4N, O, P and Q).
Figure 4. Transmuscular injury.
At day 1, most of the epithelial layer and lamina propria were absent and the superficial layer of the thyroarytenoid muscle was damaged (A). Epithelial hyperplasia and infiltration of the lamina propria by inflammatory cells were observed at day 3 (B). Granulation tissue was present at day 5 (C). At days 14 and 35, a reduction in lamina propria volume and thickening of the epithelial layer was observed (D, E). Collagen density was elevated at all time points post-injury (H–K) compared to uninjured vocal folds (L). Elastin deposition was also elevated above non-injured levels at all time points (N–Q) (200µm scale bar; original magnification 20×).
DISCUSSION
Variations in outcomes following vocal fold surgery can be attributed to, in part, differences in extent of resection6. In the present study, we proposed a surgical classification system for use in rat vocal fold wound healing studies for which the types of surgery were defined according to the depth, and anterior to posterior extent, of resection. Injury ranged from a shallow resection of the epithelium and superficial layer of the lamina propria at the midmembranous region of the vocal fold to resection of the mucosa from the anterior commissure to the vocal process of the arytenoid (. We demonstrated that the type of injury resulted in marked and consistent differences in surgical outcomes. Focal removal of the epithelium and superficial lamina propria resulted in rapid and complete healing within five days post-surgery. In contrast, healing following deeper injury was associated with a greater inflammatory response and prolonged healing; resection to the level of the vocalis muscle remained incomplete at five weeks post-injury. These differences highlight the need for consistency in injuries if surgical outcomes are to be compared across studies.
Investigators have sought to achieve consistency in injury depth across animals and studies by verifying exposure of the thyroartenoid immediately post-injury. This depth of injury corresponds best to the transmucosal injury in our proposed classification system. While this method may ensure adequate depth of injury, we showed that subtle difference in resection margins performed using an endoscopy-guided needle procedures can be performed consistency in an animal model as small as a rat, and importantly, yield different healing outcomes. The applicability of our classification system to other direct surgical techniques including laser surgery awaits investigation.
The sequence of wound healing in the vocal fold has been well-explored. The vocal fold is a trilayered structure formed by the epithelium, lamina propria, and thyroarytentoid muscle. The lamina propria is composed of cells (e.g. fibroblasts and macrophages) and an extracellular matrix composed of collagen, procollagen, and elastin among other constituents. Normal voice production requires a pliable lamina propria, which in turn, depends on the cellular and extracellular composition and organization of the lamina propria13. While we did not assess the viscoelastic properties of the scarred vocal folds, we observed changes in cell density and extracellular matrix composition consistent with altered vocal fold viscoelastic properties. Following injury, cellular infiltration and a dense and disorganized deposition of extracellular components in the lamina propria is common suggesting that our findings were consistent with injury-related changes reported elsewhere1,17, 19,22, 26. For example, we observed thickening of the epithelium and infiltration of inflammatory cells into the lamina propria post injury26. We also observed an increase in collagen deposition post-surgery beginning at day 3 post-injury in type II and III injuries. Increased collagen I and III deposition have been observed during the acute11 and chronic phases of wound healing in rat models1,17, with a peak in collagen gene expression levels three days post-injury21. These changes in the collagen density have been associated with increased stiffness13, increased phonation threshold, and reduced glottal efficiency17.
An increase in elastin staining was observed at each time point following all types of injury. This finding was unexpected as others have reported that elastin expression did not increase within three days post-injury in a rat model21,33. Further, elastin has been shown to decrease during the chronic phase of wound healing in a rabbit13 and dog34 model. Elastin is not produced after puberty in humans35. We speculate that this apparent increase in elastin was likely attributable to disorgranization and fragmentation of existing elastin3 and a shortening of elastin fibers13 observed previously in vocal folds post-injury. We further speculate that, as observed elsewhere9, variability in elastin deposition occurs regardless of the depth of vocal fold resection in humans, suggesting that individual differences in healing may contribute to outcomes.
There are several limitations to this study. One limitation is that we did not examine the rheological or vibratory properties of the tissue post-injury ex vivo. However, we examined collagen and elastin levels in the vocal fold lamina propria. As noted above, these extracellular proteins play important roles in dictating vocal fold pliability and, consequently, ease of phonation and vocal quality13.. A second limitation is that we did not include analysis of healing following an extensive, clinically relevant vocal fold resection of the mucosa and muscle. This type of surgery, a total muscular surgery, is performed clinically in cases of a malignant lesion such as T1a invasive carcinoma (Supplementary Figure 2). While this surgery could likely be performed in larger animals, we did not attempt to perform this in live rats due to the risk of extensive bleeding and consequent asphyxiation due to the small size of the rat airway. Another limitation is that we used a rat model. Rats, like mice, have an accelerated life-cycle relative to humans. Consequently, one may expect that the sequence of healing in a small animal model will be applicable to the human model, but timing will not1,11. We considered using a mouse model, however, while mice have the advantage of offering many knockout strains to study gene-related mechanisms of wound healing11, the small size of their larynx would render it challenging to create consistent and specific injury to the vocal folds. Further, the lamina propria of the rat offers greater morphologic similarity to the human larynx than mice10. A final limitation was that translation of findings to clinical cases is problematic regardless of the animal model used due to species to species variations in laryngeal anatomy and physiology. In the absence of data on human patients, it is unclear how reflective the observed histopathological changes in rats are wound healing in clinical settings. However, it has been suggested that similarities in findings across species may indicate a conserved responses to vocal fold injury across species36. Further, animal studies are essential to elucidate the differences of the damage related to the surgical extension and depth. One important reason for this is that a systemic study of injury cannot be conducted clinically. For example, the extent and depth of a procedure performed in a clinical setting is dependent on the lesion, pathophysiology, and the demands of patient. While it is difficult to extrapolate findings to human patients, an understanding of the time line for repair and the important differences in histopathological outcomes across resections types may provide insight into the importance of subtle differences in injury depth on wound healing.
CONCLUSION
Here, we proposed a system for classifying rat surgery according to the depth and extent of injury. The depth of surgical incision ranged from removal of the epithelial layer in a subepithelial injury to resection of the vocal fold mucosa and superficial layer of the vocalis muscle in a transmuscular injury. Extent of injury ranged along the anterior to posterior plane from a focal injury at the midmembranous portion of the vocal fold in a subepithelial injury to lesion along the full length of the membranous vocal fold in the other injuries. The timing and completeness of wound healing, as evaluated by vocal fold morphology and collagen and elastin levels in the lamina propria, varied markedly and consistently by injury type. Most notably, deeper injury was associated greater presence of inflammation and with longer healing time. Use of our classification system, may facilitate uniformity in injury and ease comparison of surgical outcomes across animal studies.
Supplementary Material
Rats were anesthetized and placed on an operating platform (A). Vocal folds were visualized using a 1mm steel wire laryngoscope and a 1.9mm diameter 30 degree endoscope connected to a video monitor (B). Injury was created with a 25-gauge needle (C). Larynges were excised for histological evaluation (D).
Sketch and hematoxylin and eosin staining of resection of the entire vocal fold mucosa and vocalis muscle. The red line indicates the depth and extent of injury.
Supplementary Table 1: Instrumentation and surgical margins in rat models of vocal fold reported in the literature.
ACKNOWLEDGMENTS
This work was supported by NIH-NIDCD R03 DC011355, R01 DC04336, R01 DC012773 and Fukushima Medical University. We gratefully acknowledge Drew Allen Roenneburg for his expert assistance with the histology portion of this study.
Footnotes
No author declares a conflict of interest.
Contributor Information
Mitsuyoshi Imaizumi, Department of Otolaryngology, School of Medicine, Fukushima Medical University, Fukushima City, Japan.
Susan L. Thibeault, Division of Otolaryngology – Head and Neck Surgery, Department of Surgery, University of Wisconsin – Madison, 5107 WIMR, 1111 Highland Avenue, Madison WI 53705.
Ciara Leydon, Division of Otolaryngology – Head and Neck Surgery, Department of Surgery, University of Wisconsin – Madison, 5105 WIMR, 1111 Highland Avenue, Madison WI 53705.
REFERENCES
- 1.Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–191. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BQ, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–545. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau B, Hirano S, Chan RW, Welham N, Thibeault S, Ford C, Bless D. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–124. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Thibeault SL, Klemuk SA, Chen X, Quinchia Johnson BH. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. J Voice. 2011;25:249–253. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho SH, Kim HT, Lee IJ, Kim MS, Park HJ. Influence of phonation on basement membrane zone recovery after phonomicrosurgery: a canine model. Ann Otol Rhinol Laryngol. 2000;109:658–666. doi: 10.1177/000348940010900709. [DOI] [PubMed] [Google Scholar]
- 6.Hirano S, Bless DM, Rousseau B, Welham NV, Scheidt T, Ford CN. Fibronectin and adhesion molecules on canine scarred vocal folds. Laryngoscope. 2003;113:966–972. doi: 10.1097/00005537-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau B, Sohn J, Montequin DW, Tateya I, Bless D. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann Otol Rhinol Laryngol. 2004;113:767–776. doi: 10.1177/000348940411301001. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau B, Tateya I, Lim XH, Munoz-del-Rio A, Bless D. Investigation of anti-hyaluronidase treatment on vocal fold wound healing. J Voice. 2006;20:443–451. doi: 10.1016/j.jvoice.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000 May;110(5 Pt 1):814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita M, Bless DM, Welham NV. Surgical method to create vocal fold injuries in mice. Ann Otol Rhinol Laryngol. 2009;118:131–138. doi: 10.1177/000348940911800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita M, Bless DM, Welham NV. Morphological and extracellular matrix changes following vocal fold injury in mice. Cells Tissues Organs. 2010;192:262–271. doi: 10.1159/000315476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 14.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of vocal fold extracellular matrix I: Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2005;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- 15.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of vocal fold extracellular matrix II: Collagen. Ann Otol Rhinol Laryngol. 2006;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 16.Krausert CR, Ying D, Choi SH, Hoffman MR, Jiang JJ. Effect of vocal fold injury location on vibratory parameters in excised canine larynges. Otolaryngol Head Neck Surg. 2013 Jan;148:89–95. doi: 10.1177/0194599812464336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold. In: Bless D, Abbs J, editors. Vocal fold physiology: Contemporary research and clinical issues. San Diego, CA: College Hill Press; 1983. pp. 3–21. [Google Scholar]
- 19.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 20.Li NY, Vodovotz Y, Hebda PA, Abbott KV. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol. 2010;119:412–423. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim X, Tateya I, Tateya T, Munoz-Del-Rio A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 2006;115:921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 22.Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: a rat study. Ann Otol Rhinol Laryngol. 2006;115:135–143. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]
- 23.Ohno T, French LC, Hirano S, Ossoff RH, Rousseau B. Effect of hepatocyte growth factor on gene expression of extracellular matrix during wound healing of the injured rat vocal fold. Ann Otol Rhinol Laryngol. 2008;117:696–702. doi: 10.1177/000348940811700912. [DOI] [PubMed] [Google Scholar]
- 24.Ohno T, Hirano S, Rousseau B. Gene expression of transforming growth factor-beta1 and hepatocyte growth factor during wound healing of injured rat vocal fold. Laryngoscope. 2009;119:806–810. doi: 10.1002/lary.20174. [DOI] [PubMed] [Google Scholar]
- 25.Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Current opinion in otolaryngology & head and neck surgery. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling C, Raasch JL, Welham NV. E-cadherin and transglutaminase-1 epithelial barrier restoration precedes type IV collagen basement membrane reconstruction following vocal fold mucosal injury. Cells Tissues Organs. 2011;193:158–169. doi: 10.1159/000318605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li NY, Lee BJ, Thibeault SL. Temporal and spatial expression of high-mobility group box 1 in surgically injured rat vocal folds. Laryngoscope. 2012;122:364–369. doi: 10.1002/lary.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remacle M, Eckel HE, Antonelli A, Brasnu D, Chevalier D, Friedrich G, Olofsson J, et al. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur Arch Otorhinolaryngol. 2000;257:227–231. doi: 10.1007/s004050050228. [DOI] [PubMed] [Google Scholar]
- 30.Remacle M, Van Haverbeke C, Eckel H, Bradley P, Chevalier D, Djukic V, de Vicentiis M, et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol. 2007;264:499–504. doi: 10.1007/s00405-007-0279-z. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich G, Ramacle M, Birchall M, Marie JP, Arens C. Defining phonosurgery: a proposal for classification and nomenclature by the Phonosurgery Committee of the European Laryngological Society (ELS) Eur Arch Otorhinolaryngol. 2007;264:1191–1200. doi: 10.1007/s00405-007-0333-x. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Conner N, Lee K, Leverson G, Ford CN. Laryngeal-Respiratory Kinematics are Impaired in Aged Rats. Ann Otol Rhinol Laryngol. 2002;111:684–689. doi: 10.1177/000348940211100805. [DOI] [PubMed] [Google Scholar]
- 33.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory factor profiles one hour following vocal fold injury. Annals of Otology, Rhinology, and Laryngolgoy. 2008;117:145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, Bless DM. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Moore J, Thibeault SL. Insights into the role of elastin in vocal fold health and disease. J Voice. 2012;26:269–275. doi: 10.1016/j.jvoice.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen J. Cause of vocal fold scar. Current Opinion in Otolaryngolgy & Head and Neck Surgery. 2010;18:475–480. doi: 10.1097/MOO.0b013e32833fecd1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rats were anesthetized and placed on an operating platform (A). Vocal folds were visualized using a 1mm steel wire laryngoscope and a 1.9mm diameter 30 degree endoscope connected to a video monitor (B). Injury was created with a 25-gauge needle (C). Larynges were excised for histological evaluation (D).
Sketch and hematoxylin and eosin staining of resection of the entire vocal fold mucosa and vocalis muscle. The red line indicates the depth and extent of injury.
Supplementary Table 1: Instrumentation and surgical margins in rat models of vocal fold reported in the literature.