Abstract
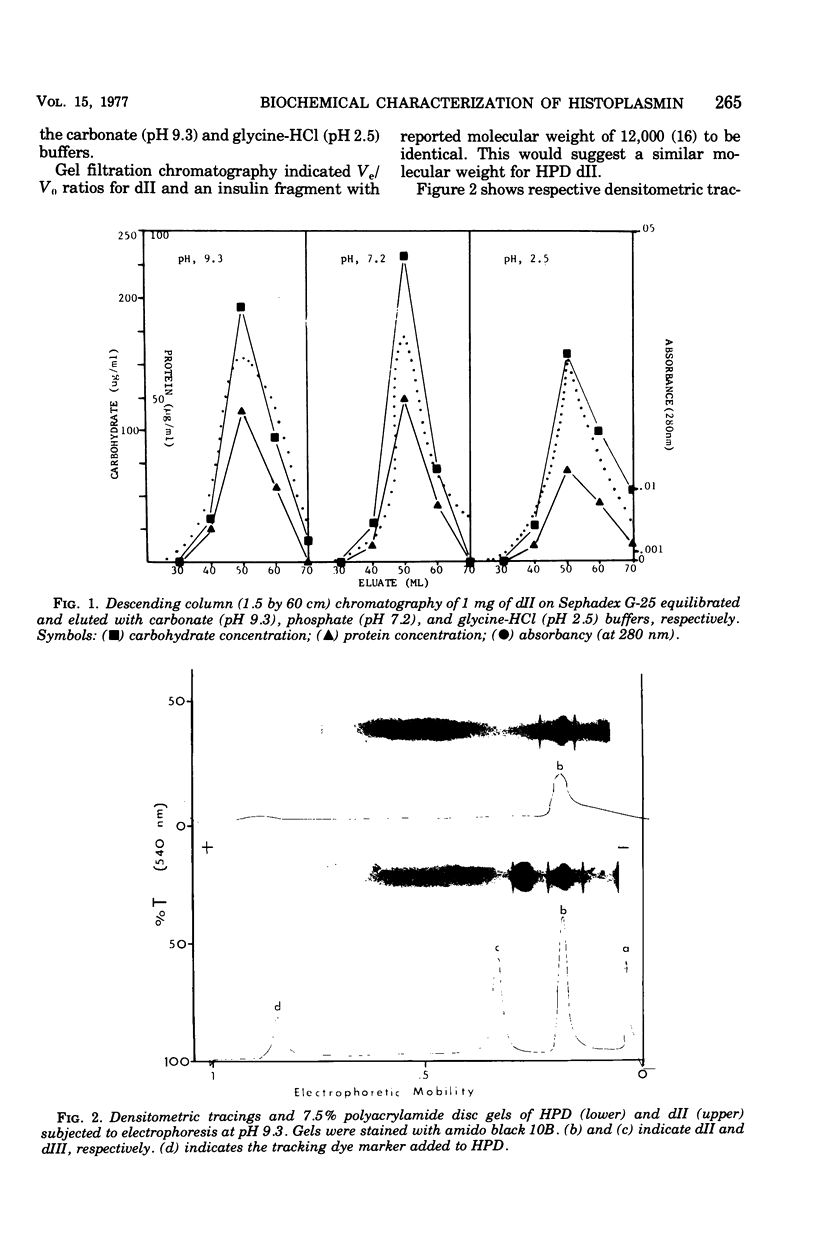
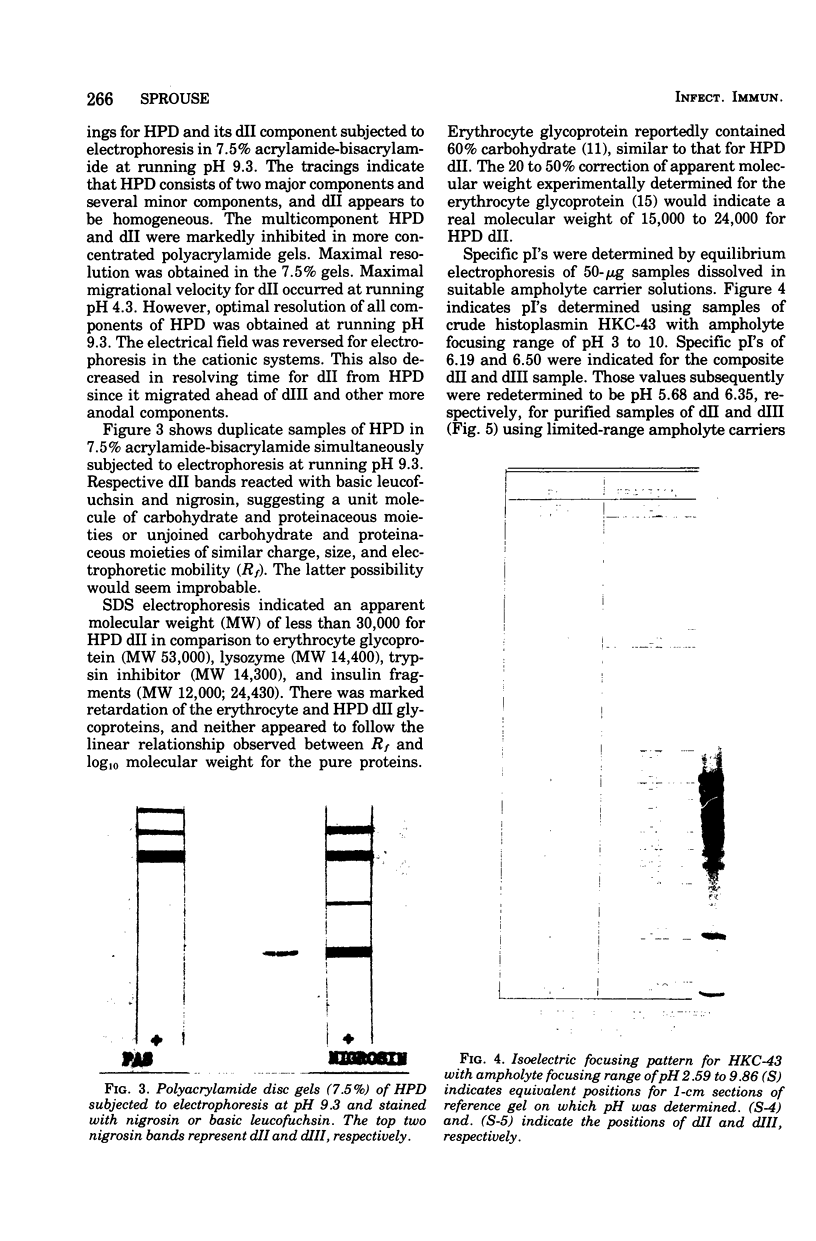
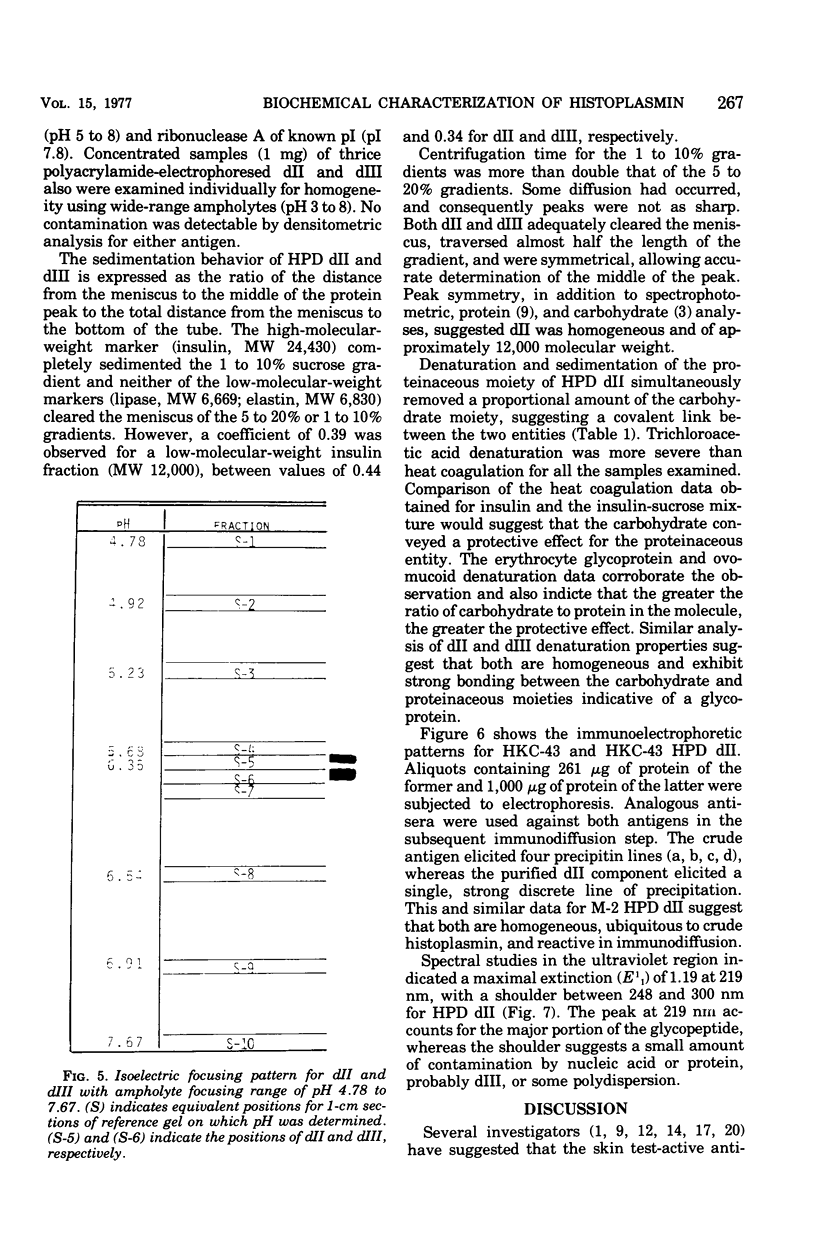
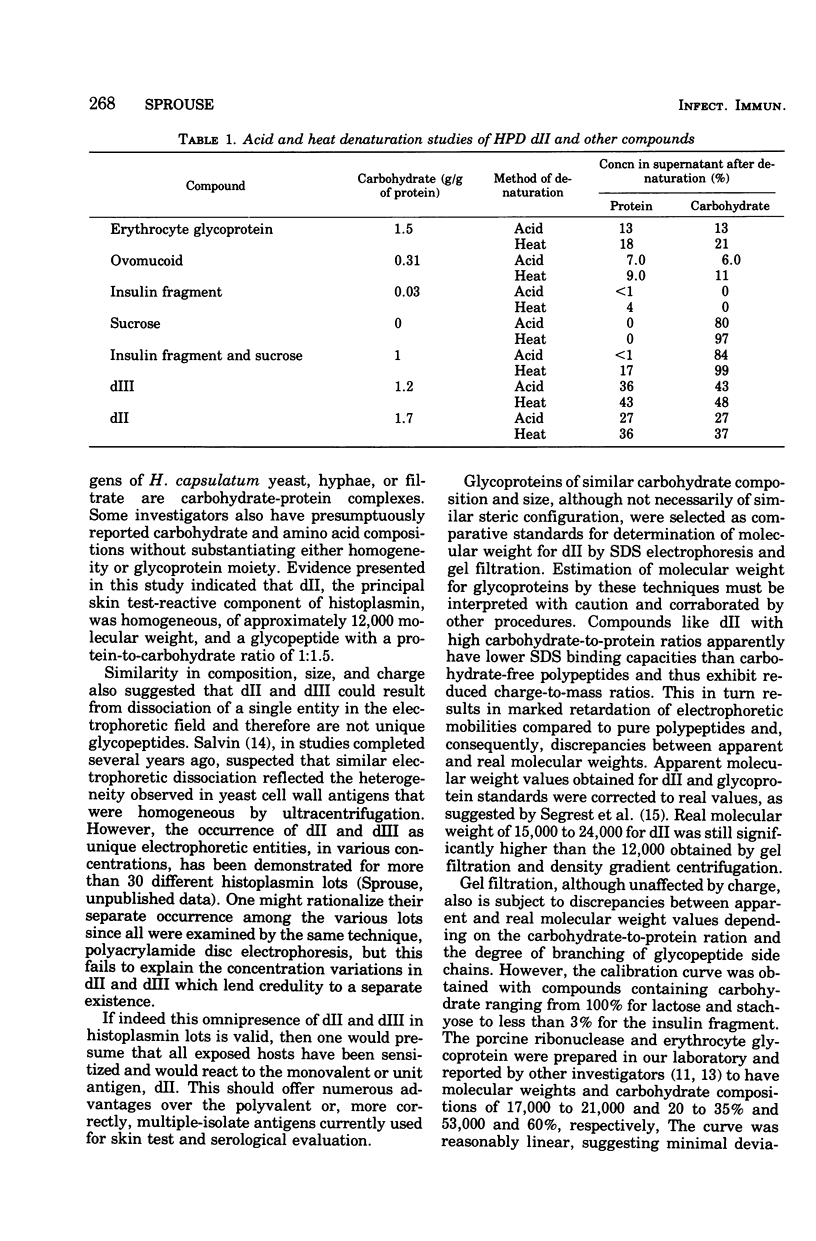
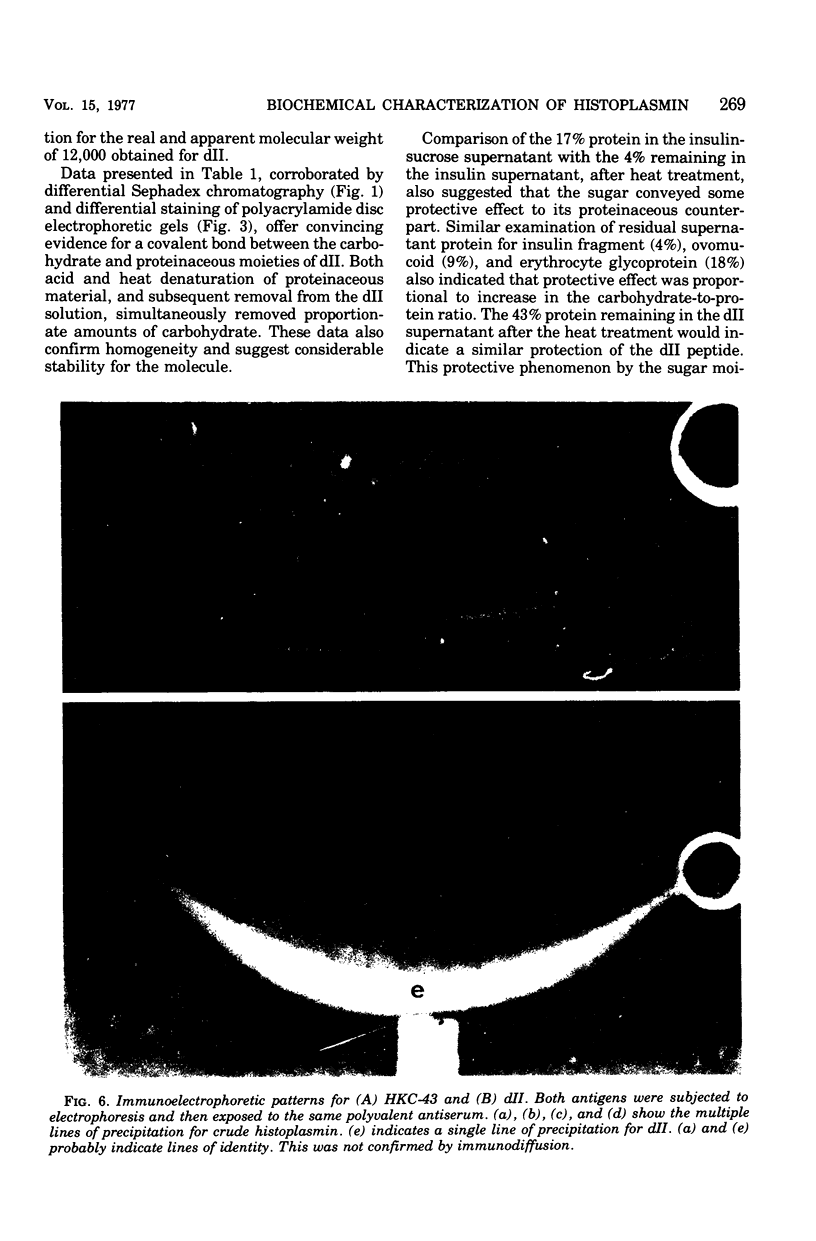
A purified component designated HPD (histoplasmin-purified derivative) dII was isolated from two different cru-e histoplasmin lots by a combination of gel filtration and polyacrylamide disc electrophoresis (Sprouse, 1969). A 0.05-mug portion of HPD dII was reactive and specific in detection of delayed hypersensitivity in guinea pigs experimentally infected with Histoplasma capsulatum. The objective of this study was to characterize this skin test-reactive component for (i) homogeneity, (ii) molecular weight, (iii) isoelectric point, and (iv) composition. Sephadex chromatography, polyacrylamide disc electrophoresis, sucrose density gradient ultracentrifugation, acid and heat denaturation, and immunoelectrophoresis indicate that HPD dII is (i) homogeneous, (ii) of approximately 12,000 molecular weight, and (iii) a glycopeptide of approximately 60% carbohydrate and 40% proteinaceous composition. The marked acid and heat stability exhibited by the compound probably is attributable to the prominent carbohydrate moiety in the molecule. Isoelectric focusing indicated an isoelectric point of 5.68. This would suggest that dII is an acidic compound with either predominance of acidic amino acid residues in the molecule or, more probably, an abundance of electron donors in the carbohydrate moiety. In summary, HPD dII appears to be a glycopeptide of approximately 12,000 molecular weight, reactive in elicitation of delayed hypersensitivity of histoplasmosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catsimpoolas N. Micro isoelectric focusing in polyacrylamide gel columns. Anal Biochem. 1968 Dec;26(3):480–482. doi: 10.1016/0003-2697(68)90219-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Griffith A., Catsimpoolas N. General aspects of analytical isotachophoresis of proteins in polyacrylamide gels. Anal Biochem. 1972 Jan;45(1):192–201. doi: 10.1016/0003-2697(72)90019-x. [DOI] [PubMed] [Google Scholar]
- KNIGHT R. A., MARCUS S. Polysaccharide skin test antigens derived from Histoplasma capsulatum and Blastomyces dermatitidis. Am Rev Tuberc. 1958 Jun;77(6):983–989. doi: 10.1164/artpd.1958.77.6.983. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Terry R. T., Schubert J. H., McLaughlin D. Effects of a single histoplasmin skin test on the serological diagnosis of histoplasmosis. J Bacteriol. 1967 Oct;94(4):798–803. doi: 10.1128/jb.94.4.798-803.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKOWITZ H. POLYSACCHARIDE ANTIGENS FROM HISTOPLASMA CAPSULATUM. Proc Soc Exp Biol Med. 1964 Mar;115:697–700. doi: 10.3181/00379727-115-29010. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold V. N., Dunne F. T., Wriston J. C., Schwarz M., Sarda L., Hirs C. H. The isolation of porcine ribonuclease, a glycoprotein, from pancreatic juice. J Biol Chem. 1968 Dec 25;243(24):6482–6494. [PubMed] [Google Scholar]
- SALVIN S. B., SMITH R. F. Antigens from the yeast phase of Histoplasma capsulatum. III. Isolation, properties, and activity of a protein-carbohydrate complex. J Infect Dis. 1959 Jul-Aug;105(1):45–53. doi: 10.1093/infdis/105.1.45. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Sprouse R. F., Goodman N. L., Larsh H. W. Fractionation, isolation and chemical characterization of skin test active components of histoplasmin. Sabouraudia. 1969 Feb;7(1):1–11. doi: 10.1080/00362177085190021. [DOI] [PubMed] [Google Scholar]
- Sprouse R. F. Purification of histoplasmin purified derivative. I. Disc electrophoretic separation of skin test-reactive components. Am Rev Respir Dis. 1969 Nov;100(5):685–690. doi: 10.1164/arrd.1969.100.5.685. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Monod J. On the effect of divalent cations and protein concentration upon renaturation of beta-galactosidase from E. coli. Biochem Biophys Res Commun. 1969 Apr 10;35(1):35–42. doi: 10.1016/0006-291x(69)90479-3. [DOI] [PubMed] [Google Scholar]