Abstract
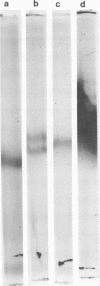
The avirulent Madrid E strain of Rickettsia prowazekii cultivated in chicken yolk sacs could be purified successfully with a Renografin density gradient method developed previously for Rickettsia typhi. Recovery during purification, viability, and lack of contamination with host cell components were similar for the two species, although yields of R. prowazekii per yolk sac were lower. Purified typhus rickettsiae provided satisfactory antigens in the complement fixation, Ouchterlony double-diffusion, and microagglutination tests. The retention of the typhus soluble group antigen during purification was readily demonstrated by complement fixation tests. However, removal of the soluble group antigen by ether treatment was not always adequate for the demonstration of type-specific particulate antigens. Heat-killed R. prowazekii cells gave higher serum microagglutination titers than untreated or formalized cells, a difference was noted for R. typhi cells. Although the protein profiles of whole cells and extracts of R. typhi and R. prowazekii on sodium dodecyl sulfate-polyacrylamide gels were relatively similar, a small but reproducible, difference in the electrophoretic mobilities of their malate dehydrogenases was detected. Purification of typhus rickettsiae on Renografin gradients has no apparent adverse effects on their metabolic or antigenic properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Pickens E. G., Lackman D. B. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J Bacteriol. 1967 Jul;94(1):260–262. doi: 10.1128/jb.94.1.260-262.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balayeva N. M., Gulevskaya S. A. Comparative characteristics of "ether" and "non-ether" soluble ricketssia prowazekii antigens and electron microscopy findings on the morphology of the rickettsiae during isolation of the antigens. J Hyg Epidemiol Microbiol Immunol. 1972;16(1):92–100. [PubMed] [Google Scholar]
- Barker L. F., Patt J. K. Production of rickettsial complement-fixing antigens in tissue culture. J Immunol. 1968 Apr;100(4):821–824. [PubMed] [Google Scholar]
- Bozeman F. M., Masiello S. A., Williams M. S., Elisberg B. L. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975 Jun 12;255(5509):545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- Brezina R. New advances in the study of rickettsial antigens. Zentralbl Bakteriol Orig. 1968 Apr;206(3):313–321. [PubMed] [Google Scholar]
- Fink M. A., Feller W. F., Sibal L. R. Methods for detection of antibody to the mammary tumor virus. J Natl Cancer Inst. 1968 Dec;41(6):1395–1400. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Golinevitch H. M., Voronova Z. A. The superficial protective antigen of R. prowazeki. J Hyg Epidemiol Microbiol Immunol. 1968;12(4):413–419. [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Palmer E. L., Martin M. L., Mallavia L. Ultrastucture of the surface of Rickettsia prowazeki and Rickettsia akari. Appl Microbiol. 1974 Oct;28(4):713–716. doi: 10.1128/am.28.4.713-716.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for May 31, 1946. Public Health Rep. 1946 May 31;61(22):761–800. [PMC free article] [PubMed] [Google Scholar]
- Reiss-Gutfreund R. J., Cappucinelli P., Cavallo G. The soluble antigens of Rickettsia prowazeki, R. typhi and R. canada. Investigation of their interrelationship by various serological methods. Z Immunitatsforsch Exp Klin Immunol. 1975 Feb;148(4):315–329. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Tzianabos T., Palmer E. L., Obijeski J. F., Martin M. L. Origin and structure of the group-specific, complement-fixing antigen of Rickettsia rickettsii. Appl Microbiol. 1974 Sep;28(3):481–488. doi: 10.1128/am.28.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Selection of an erythromycin-resistant strain of Rickettsia prowazekii. Am J Hyg. 1960 May;71:292–298. doi: 10.1093/oxfordjournals.aje.a120113. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]