Abstract
Contest decisions are influenced by the outcomes of recent fights (winner–loser effects). Steroid hormones and serotonin are closely associated with aggression and therefore probably also play important roles in mediating winner–loser effects. In mangrove rivulus fish, Kryptolebias marmoratus, individuals with higher testosterone (T), 11-ketotestosterone and cortisol levels are more capable of winning, but titres of these hormones do not directly mediate winner–loser effects. In this study, we investigated the effects of winning/losing experiences on brain expression levels of the receptor genes for androgen (AR), oestrogen α/β (ERα/β), glucocorticoid (GR) and serotonin (5-HT1AR). The effect of contest experience on AR gene expression depended on T levels: repeated losses decreased, whereas repeated wins increased AR gene expression in individuals with low T but not in individuals with medium or high T levels. These results lend strong support for AR being involved in mediating winner–loser effects, which, in previous studies, were more detectable in individuals with lower T. Furthermore, the expression levels of ERα/β, 5-HT1AR and GR genes were higher in individuals that initiated contests against larger opponents than in those that did not. Overall, contest experience, underlying endocrine state and hormone and serotonin receptor expression patterns interacted to modulate contest decisions jointly.
Keywords: winner/loser effect, testosterone, hormone receptor, 5-HT1A receptor, Kryptolebias marmoratus
1. Introduction
Recent victories and defeats affect an individual's behaviour in and its tendency to win future contests [1]. Although winner–loser effects are strongly conserved from invertebrates to humans, their underlying physiological mechanisms remain unclear [2]. The neuroendocrine system plays an important role in aggression and dominance status [3–5]. Individuals with more testosterone (T) are more aggressive and achieve dominance [6], effects that disappear with loss of androgen receptor (AR) function [7]. Oestradiol (E2) supplementation promotes male-like aggressiveness in female mice [8], an effect driven through oestrogen receptor α, but not receptor β [9,10]. Chronic cortisol (F) treatment and glucocorticoid receptor (GR) antagonism inhibit aggression and predispose subordination [11,12], whereas acute increases in glucocorticoids trigger intense aggression [13,14]. Acute serotonin (5-HT) treatment and 5-HT1A receptor activation reduce aggressive behaviour [15]. Close associations between steroid hormones, the serotonergic system and contest decisions [16] suggest that these mechanisms might be involved in translating fighting experience into future changes in contest performance.
In California mice (Peromyscus californicus), individuals with more wins showed significantly stronger winner effects and higher post-encounter T than those with fewer [17]. Contest experience and post-experience T administration contributed additively to winner effects [6,18], and brain AR gene/protein expression increased in mice that won contests in their home cages [19]. In cichlid fish, AR antagonism blocked winner effects, but androgen administration failed to reverse loser effects [2], suggesting different underlying mechanisms. However, post-contest T administration reversed the loser effect in Japanese quail, Coturnix japonica [20].
Mangrove rivulus fish, Kryptolebias marmoratus, display winner and loser effects that last for 48 h [21] or longer [22], and their contest behaviour is highly correlated with pre-contest levels of T, 11-ketotestosterone (KT) and F [23,24]. Furthermore, the fish's propensity to adjust contest decisions after wins and losses depends on its hormonal state: individuals with lower levels of F, T and KT are more likely than others to change contest behaviour after winning/losing experiences [25]. Winning/losing experiences, however, change the fish's behaviour without affecting the levels of these hormones [25], indicating that these hormones do not, alone, precipitate winner or loser effects. We therefore hypothesized that fighting experience might drive changes in the fish's behaviour by triggering changes in brain steroid and serotonin receptor gene expression. In this study, we tested this hypothesis by examining changes in brain receptor gene expression after randomly assigned winning, losing or no-contest experiences and whether these changes might be dependent on baseline steroid hormone levels.
2. Material and methods
(a). Experimental design
This experiment aimed to test whether steroid hormone/serotonin receptor expression might be associated with the winner/loser effects observed in K. marmoratus. We randomly selected individuals to receive forced winning (W), losing (L) or no-contest experiences (N), following procedures that have repeatedly produced winner and loser effects in the fish [21,23]. Loser effects always are readily observed in K. marmoratus, but winner effects often are less detectable unless the fish have been through multiple training experiences [25], so we gave half the fish one experience only (1W, 1L and 1N) and the other half three similar experiences (3W, 3L and 3N), a total of six experience treatments. The effects of winning/losing on future contest performance and outcome are strongest 0–3 h after the initial experience and decay after 48 h [21], so we separated the fish into different decay-time treatment groups and tested receptor expression 0, 3 or 48 h after experience. Six experience treatments × three time-decay treatments make 18 treatments. After the relevant period of time, we decapitated the fish and extracted their brains to quantify receptor gene expression levels using quantitative PCR (qPCR).
We quantified expression of AR, ERα/β, GR and 5-HT1AR genes because all are closely associated with dominance and/or aggression. We measured experimental individuals' pre-experience hormone levels (F and T) because a previous study showed that the significance of winner–loser effects in this fish depends on levels of steroid hormones [25]. We also recorded the experimental individuals' behaviour during experience training to include these data in our statistical models together with fishes' size and isogenic lineage (see below). All fish used in this study had experience of interacting with other conspecifics and had subsequently been isolated for at least one month to reduce any impact that this might have had on the current experiment. The study used 295 fish randomly assigned to the 18 treatments (n = 16 or 17 per treatment).
(b). Study organism
Mangrove rivulus K. marmoratus is a self-fertilizing hermaphroditic fish that inhabits mangrove ecosystems ranging from southeastern Brazil, Venezuela, the Caribbean and Yucatan to Florida and the Bahamas [26]. It is aggressive in the field and the laboratory [27]. This study used individuals of three isogenic lineages of K. marmoratus from different geographical areas (DAN2K: Dangria, Belize, collected in 2000; RHL: San Salvador, Bahamas, collected in 1997; SLC8E: St. Lucie County, FL, USA, collected in 1995), all of which were descendants of individuals originally collected from the field by Dr D. Scott Taylor. Fish were isolated on the day of hatching and kept individually in 13 × 13 × 10 cm3 translucent plastic containers (maintenance container). Every container was filled with 800 ml of 25 ppt synthetic sea water (Instant Ocean powder) and labelled with a unique code for individual identification. Fish were maintained at 25 ± 2°C on a 12 L : 12 D photoperiod and fed 2 ml newly hatched brine shrimp (Artemia) nauplii at 1500 h every day.
(c). Experimental procedures
On day 1, before animals were exposed to any of the treatments, T and F were measured as indicators of the fishes' baseline endocrine status (pre-experience hormone levels). Focal individuals pre-assigned to have three experiences (3W, 3L and 3N) received one winning, losing or no-contest experience on each of days 2, 3 and 4. Focal individuals pre-assigned to receive one experience (1W, 1L and 1N) received their single experience on day 2. Immediately after completion of experience training, individuals assigned to the 0 h decay treatment were decapitated and their heads preserved in RNAlater (Applied Biosystems/Ambion Inc., TX, USA) for 24 h at 4°C prior to storage at −80°C for subsequent examination of receptor gene expression. Individuals assigned to the 3 and 48 h decay-time treatments were returned to their maintenance containers after completion of experience training, decapitated 3 and 48 h afterwards and their heads preserved as above.
(d). Collection, extraction and assay of hormones
Procedures for hormone sample collection and analysis follow Earley & Hsu [23]. Detailed procedures are described in the electronic supplementary material, S1. All hormone data are presented as picogram per millilitre.
(e). Providing a losing/winning experience
To ensure that fish (standard length (SL), mean ± s.d. = 29.59 ± 1.00 mm) lost or won as determined, we fought them against much larger/smaller (difference more than 2 mm) standard winners/losers that had won/lost several contests against similar-sized opponents from the same isogenic lineage. Individuals assigned to receive three contest experiences (3L and 3W) were fought against three different standard winners/losers to avoid problems of individual recognition. For experience training, fish were placed in one of two symmetrical compartments (randomly selected) of a 12 × 8 × 20 cm3 aquarium containing water 13 cm deep and 2 cm of gravel, separated by an opaque partition from a standard winner/loser in the other compartment. All fish were given 30 min to acclimate before the partition was removed, at which time the experimental individual was allowed to interact with its trainer. A losing experience was completed when the experimental individual retreated from the standard winner's display/attack and quickly swam away. A winning experience was completed when the standard loser retreated from the experimental individual's display/attack and quickly swam away. The opaque partition was replaced to separate the experimental fish and its trainer as soon as the experimental fish received its pre-assigned experience. All fish were returned to their maintenance containers after experience training. Experimental fish received their pre-assigned winning or losing experience quickly (three-experiences treatment: median = 51.0, 25.5, 26.5 s for the 1st, 2nd and 3rd losing experiences, respectively; 50.5, 40.5, 45.0 s for the 1st, 2nd and 3rd winning experience, respectively; one-experience treatment: median = 62.0 s for losing experience; 60.5 s for winning experience). The 3N and 1N individuals were treated in exactly the same way as the others on days 2–4, except with no opponent in the standard aquarium, so that they received the same amount of handling as the other experimental individuals.
To explore possible relationships between the fish's behaviour and receptor gene expression levels, we recorded whether the experimental fish initiated contest interactions in the first experience training by orienting its head towards and/or approaching the standard trainer fish. Experience training sessions were videotaped.
(f). Quantifying receptor gene expression levels
We developed nested sets of primers for the receptor genes by downloading known sequences from the NCBI website (www.ncbi.nih.gov). These sequences were aligned using Clustal X, and primers were designed from highly homologous regions of the alignment. The forward and reverse primers that we used in polymerase chain reaction (PCR) and qPCR are listed in the electronic supplementary material, table S2. PCR was conducted with an Eppendorf Mastercycler Gradient, using the 5 PRIME HotMaster Mix (5 PRIME Inc., MD, USA) and the temperature gradient feature to maximize amplification and efficiency.
PCR and RT-PCR were used to target the receptor gene sequences. We then quantified gene expression using qPCR performed on the Mastercycler ep realplex System with SYBR (Kapa Biosystems, Inc., MA, USA) green according to the manufacturer's instructions. Detailed procedures are provided in the electronic supplementary material, S3. The RPL8 gene was used as a control gene to normalize expression levels between samples, following a previous study [28]. All data were expressed relative to the RPL8 gene to normalize for any difference in reverse transcriptase efficiency. Threshold cycle (Ct) values were obtained from Mastercycler ep realplex System software (Eppendorf, NY, USA) and used to calculate delta threshold cycle (ΔCt) values (ΔCt = Cttarget gene − Ctcontrol gene) of each sample [29]. ΔCt values are negatively correlated with relative gene expression: higher ΔCt values indicate lower receptor gene expression levels. We therefore used –ΔCt values to conduct all statistical analyses such that higher values indicate higher receptor gene expression levels.
(g). Data analysis
We first used Pearson's pairwise correlations to measure the overall relationships between pre-experience hormone levels, between post-experience receptor gene expression levels and between pre-experience hormone levels and post-experience receptor gene expression levels. The distributions of the pre-experience hormone levels were very skewed and were improved through natural-log (ln) transformation.
(i). Effects of experience type and decay time on receptor gene expression
We used general linear models to examine whether the focal individuals' post-experience receptor gene expression levels varied with their contest experience × decay-time treatments. Hormone levels (ln-transformed), SL and lineage were included in the model as control factors. We included interactions between experience treatments and these two hormones in the models because the fish's response to winning and losing experiences is influenced by its levels of T and F [25].
(ii). Relationships between aggressiveness and receptor gene expression
The residuals of the general linear models then were analysed to examine whether a significant part of the unexplained variance in experimental individuals' receptor gene expression levels was related to their behaviour during experience training. We compared the residuals of receptor gene expression levels of individuals allocated to losing experience treatments (3L and 1L combined) between those that initiated and did not initiate contest interactions with standard winners in the first training session (independent t-tests). These analyses were not suitable for individuals that were assigned to receive winning experiences (3W and 1W) because the standard losers with which they interacted were trained to be submissive and almost never initiated contest interactions. Nor could these analyses be used for individuals assigned to receive no fighting experiences (3N and 1N) that had no opponent with which to interact.
We used SAS Enterprise Guide (v. 5.1; SAS Institute Inc., Cary, NC, USA) for the analysis of general linear models (Proc GLM) and JMP (v. 8; SAS Institute Inc.) for all the other statistical analyses in this study.
3. Results
(a). Relationships between pre-experience hormone levels and post-experience receptor gene expression levels
Receptor gene expression levels were highly correlated with each other (table 1): ERα, ERβ, GR and 5-HT1AR gene expression were correlated positively with each other (r ≥ 0.715, p < 0.001); AR gene expression was correlated negatively with that of 5-HT1AR (r = −0.155, p = 0.008) and had no significant relationship with that of ERα, ERβ or GR. Cortisol levels were positively correlated with AR gene expression (r = 0.125, p = 0.032). Testosterone levels were positively correlated with expression of all receptor genes (r ≥ 0.142, p ≤ 0.015), although the positive relationship with 5-HT1AR did not reach significance (r = 0.112, p = 0.054). Testosterone levels also were positively correlated with F levels (r = 0.330, p < 0.001).
Table 1.
Pairwise correlations between receptor expression levels, between hormone levels and between hormone levels and receptor expression levels. (The mean values (±s.e.) of the relative receptor gene expression levels (−ΔCt) and the ln-transformed hormone levels are represented next to the label for each receptor and hormone on the top row of the table. AR, androgen receptor; ERα, oestrogen receptor α; ERβ, oestrogen receptor β; GR, glucocorticoid receptor, 5-HT1AR, serotonin 1A receptor; F, cortisol; T, testosterone.)
| receptor/hormone (mean ± s.e.m.) | AR (−10.26 ± 1.01) | ERα (−1.59 ± 1.39) | ERβ (−1.41 ± 1.41) | GR (−2.29 ± 1.12) | 5-HT1AR (−0.95 ± 1.32) | F (4.85 ± 0.55) | T (6.53 ± 0.78) |
|---|---|---|---|---|---|---|---|
| AR |
r = 0.025 p = 0.674 |
r = −0.031 p = 0.595 |
r = −0.098 p = 0.095 |
r = −0.155 p = 0.008** |
r = 0.125 p = 0.032* |
r = 0.162 p = 0.005** |
|
| ERα |
r = 0.962 p < 0.001*** |
r = 0.719 p < 0.001*** |
r = 0.796 p < 0.001*** |
r = −0.046 p = 0.427 |
r = 0.325 p < 0.001*** |
||
| ERβ |
r = 0.715 p < 0.001*** |
r = 0.797 p < 0.001*** |
r = −0.090 p = 0.124 |
r = 0.313 p < 0.001*** |
|||
| GR |
r = 0.931 p < 0.001*** |
r = −0.055 p = 0.347 |
r = 0.142 p = 0.015* |
||||
| 5-HT1AR |
r = −0.090 p = 0.121 |
r = 0.112 p = 0.054 |
|||||
| F |
r = 0.330 p < 0.001*** |
*p < 0.05; **p ≤ 0.01; ***p ≤ 0.001.
The three lineages of individuals used for the study did not differ significantly in their levels of T or F nor in their expression of any of the five receptor genes (F2,292 ≤ 1.03, p ≥ 0.359).
(b). Effect of experience type and decay time on post-experience receptor gene expression
Of the five receptor genes examined, only the expression of the AR gene was influenced by contest experience type (table 2), and the influence depended on levels of T (experience × T, p < 0.001). To illustrate the complex interaction between contest experience and T on AR gene expression, we grouped the focal individuals into those having low (less than or equal to 33.3 percentile), medium (33.3–66.6 percentile) and high (more than or equal to 66.6 percentile) T levels, and showed how experience type affects AR gene expression for these three groups of individuals (figure 1). Contest experience significantly influenced AR gene expression for experimental individuals with low levels of T (F5,91 = 3.38, p = 0.008). The main difference was between individuals with low levels of T in the 3L and 3W treatments: individuals with three losing experiences had significantly lower AR gene expression levels than those with three winning experiences (Tukey pairwise comparisons, p = 0.003). Contest experience did not have any significant effect on AR expression in individuals with medium T levels (F5,93 = 1.48, p = 0.205). The expression level of the AR gene in individuals with high T levels was significantly affected by contest experience (F5,93 = 6.48, p < 0.001): average AR gene expression was lower in each of the experience treatment groups given three training experiences than in those given one training experience, although only the 3L group showed significantly lower AR expression than the one-experience groups (Tukey pairwise comparisons, p < 0.002).
Table 2.
Influence of experience type and decay time on post-experience receptor gene expression levels (−ΔCt). (General linear models evaluated the effect of experience type, decay time, hormone levels and interactions between experience type, decay time and hormone levels on receptor gene expression, controlling for SL and lineage. AR, androgen receptor; ERα, oestrogen receptor α; ERβ, oestrogen receptor β; GR, glucocorticoid receptor, 5-HT1AR, serotonin 1A receptor; F, cortisol; T, testosterone.)
| variable | d.f. | AR |
ERα |
ERβ |
GR |
5-HT1AR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b ± s.e. | F | p | b ± s.e. | F | p | b ± s.e. | F | p | b ± s.e. | F | p | b ± s.e. | F | p | ||
| experience type | 5 | 1.32 | 0.254 | 0.71 | 0.618 | 0.87 | 0.502 | 1.72 | 0.129 | 1.74 | 0.127 | |||||
| decay time | 2 | 3.04 | 0.050* | 2.39 | 0.094 | 2.12 | 0.122 | 2.40 | 0.093 | 4.15 | 0.017* | |||||
| F | 1 | 0.26 ± 0.11 | 5.38 | 0.021* | −0.39 ± 0.16 | 6.08 | 0.014* | −0.52 ± 0.16 | 10.86 | 0.001* | −0.18 ± 0.13 | 1.87 | 0.173 | −0.32 ± 0.15 | 4.24 | 0.041* |
| T | 1 | 0.11 ± 0.08 | 2.01 | 0.158 | 0.68 ± 0.11 | 38.33 | < 0.001* | 0.72 ± 0.11 | 41.56 | <0.001* | 0.27 ± 0.09 | 8.63 | 0.004* | 0.30 ± 0.11 | 7.56 | 0.006* |
| exp × time | 10 | 0.75 | 0.679 | 0.69 | 0.733 | 0.54 | 0.863 | 0.70 | 0.721 | 0.61 | 0.801 | |||||
| exp × F | 5 | 2.04 | 0.073 | 1.02 | 0.409 | 0.68 | 0.639 | 1.51 | 0.186 | 1.06 | 0.385 | |||||
| exp × T | 5 | 4.33 | <0.001* | 1.19 | 0.313 | 1.37 | 0.235 | 1.30 | 0.263 | 2.18 | 0.056 | |||||
| SL | 1 | −0.10 ± 0.06 | 2.51 | 0.115 | −0.01 ± 0.09 | 0.00 | 0.953 | 0.03 ± 0.09 | 0.09 | 0.766 | 0.17 ± 0.07 | 5.73 | 0.017* | 0.19 ± 0.09 | 4.85 | 0.029* |
| lineage | 2 | 0.46 | 0.632 | 0.44 | 0.648 | 0.42 | 0.658 | 0.43 | 0.648 | 0.16 | 0.855 | |||||
*p < 0.05.
Figure 1.
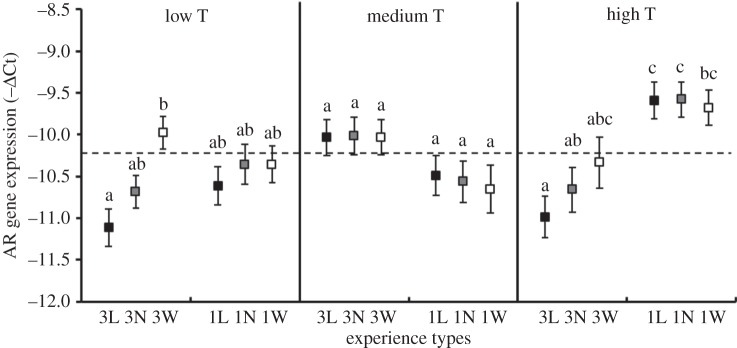
The influence of experience types on post-experience AR gene expression (−ΔCt; mean ± s.e.) for individuals with low (less than or equal to 33.3 percentile), medium (33.3–66.6 percentile) and high (more than or equal to 66.6 percentile) levels of pre-experience T. Different lowercase letters indicate significant differences between treatments (Tukey pairwise comparisons). The dotted line is the overall mean level of AR expression. Filled squares, losing experience; shaded squares, no experience; open squares, winning experience. −ΔCt equates to positive gene expression levels, so the less negative values of −ΔCt towards the top of this graph equate to higher levels of gene expression.
Decay time affected expression of AR and 5-HT1AR genes independently of the experience treatments. The expression level of the AR gene was significantly higher 48 h after experience training than 0 h after experience training (Tukey pairwise comparison, 48 h versus 0 h: p = 0.050, figure 2a); 5-HT1AR expression was significantly higher after 0 h than after 3 or 48 h (Tukey pairwise comparison, p = 0.013 and 0.048, respectively, figure 2b). These trends were independent of contest experience (non-significant experience × decay-time interactions, p ≥ 0.679), suggesting a possible handling effect.
Figure 2.
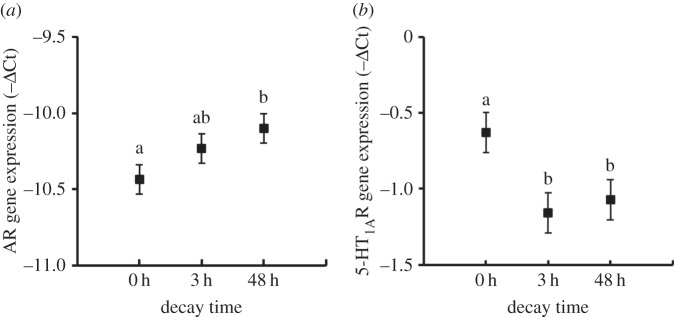
The influence of decay time on post-experience (a) AR and (b) 5-HT1AR gene expression (−ΔCt; mean ± s.e.). Different lowercase letters indicate significant differences between treatments (Tukey pairwise comparisons).
(c). Relationship between aggressiveness in first experience training and post-experience receptor gene expression levels
Of the fish that received losing experiences (1L and 3L), those that initiated aggressive displays against their larger trainers during the first training session had significantly higher residual ERα (t97 = 3.66, p < 0.001), ERβ (t97 = 3.36, p = 0.001), GR (t97 = 3.72, p < 0.001) and 5-HT1AR (t97 = 3.50, p < 0.001) gene expression than those that did not (figure 3), after taking account of all other factors in the linear model. This indicates that individuals' pre-experience aggressiveness contributed to variation in post-experience expression of these genes, with aggressive individuals having higher expression. The relationship between initiating displays and residual AR gene expression was not significant (t97 = 0.07, p = 0.944).
Figure 3.
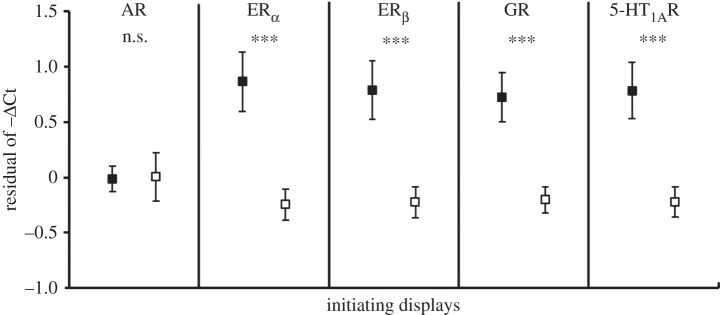
The relationship between display behaviour towards a standard winner during the first losing training experience and the residual (mean ± s.e.) of post-experience receptor gene expression (−ΔCt) from the linear model. n.s., non-significant; ***p ≤ 0.001 (two-tailed t-test). Filled squares, display-initiators; open squares, non-display-initiators.
4. Discussion
(a). Effect of winning–losing experience on post-experience receptor gene expression levels
The difference in AR gene expression caused by multiple winning and multiple losing experiences was significant only in fish with low baseline T, consistent with winner–loser effects in previous research [25], which were stronger in individuals with lower baseline T and F. This similarity—AR gene expression and subsequent behaviour both altered in fish with lower T—strongly supports AR being an important mechanism mediating winner–loser effects. In this fish, T correlates positively with the ability to win [23]. Fight outcomes could provide individuals with information about their relative fighting ability in the local population [30,31]. Because the costs of engaging in contests are probably more variable for individuals with poor fighting ability (low baseline T), it may pay them to monitor recent fights more closely and to modulate neuroendocrine substrates in ways that produce the most appropriate behavioural responses given the updated information.
Changes in both behaviour and AR gene expression levels in response to contest experience also depended on context in territorial California mice (P. californicus). Mice with three winning experiences in their home cages displayed strong winner effects and increases in AR expression in brain regions involved in motivation and reward (nucleus accumbens, ventral tegmental area); those with winning experiences in unfamiliar cages did not [19]. The different responses to winning by mice in different locations and by fish with different T levels indicate that winner–loser effects are tuned by an individual's internal state and extrinsic factors.
In our study, individuals given one winning and one losing experience did not differ in AR gene expression (regardless of T), despite the fact that one experience causes significant winner and loser effects in the fish [21,22]. This could be the result of our smaller sample size (N = 50–60 per treatment in previous studies [21,22] versus N = 11–21 per bar in figure 1), or it might be because whole-brain assays like ours cannot easily detect moderately increased gene expression in a specific brain region. The latter explanation supports the need to further develop techniques such as brain microdissection and regional or single neuron PCR [32] in small fishes. The level of AR gene expression may also not be the only physiological mechanism mediating winner–loser effects in this species. Neuropeptides such as vasotocin and isotocin are higher in aggressive fish [33,34], and vasopressin receptor (V1aR) binding is enhanced in the ventromedial hypothalamus of hamsters that experience repeated victories [35]. Central administration of corticotropin-releasing factor (CRF) influences attack frequency and aggressiveness in fish [36], and CRF receptors modulate sensitivity to social defeat in Syrian hamsters (Mesocricetus auratus) [37], perhaps by affecting serotonergic neurotransmission [38]. The involvement of neuropeptides in winner–loser effects therefore merits further investigation.
(b). Relationships between aggressiveness and receptor gene expression levels
Individuals that initiated aggression against their larger opponents in their first losing experience had higher levels of ERα, ERβ, GR and 5-HT1AR gene expression than others in our residual analysis. These receptors are therefore positively associated with aggressiveness, despite the fact that their expression did not vary with winning or losing experiences. This is consistent with findings in some other species. Male mice with their ERα gene knocked-out were less aggressive than others, but those with their ERβ gene knocked-out were not [39]. Dominant African cichlid (Astatotilapia burtoni) males expressed more ERβ (but not ERα) mRNA in the anterior brain than subordinates [40]. Glucocorticoid receptor expression usually correlates with stress rather than aggressiveness [41,42], but blocking GR by mifepristone reduced aggressive attacks/displays in the early stages of lizard (Anolis carolinensis) fights [12], suggesting a positive relationship with aggressiveness. Serotonin 1A (5-HT1A) receptors can function as post-synaptic receptors or as autoreceptors (e.g. in the dorsal raphe nucleus). Research in rodents indicates that post-synaptic and autoreceptor 5-HT1AR sensitivity might be negatively and positively correlated, respectively, with aggressiveness [43,44]. Losing experiences also cause decreased 5-HT1AR autoreceptor expression in the dorsal raphe nucleus of Syrian hamsters [45]. Our study examined the whole brain and did not distinguish between these two types of 5-HT1AR; it may be that aggressive individuals had higher autoreceptor gene expression levels in pre-synaptic neurons than non-aggressive individuals.
It is intriguing that AR expression levels varied with winning/losing experiences for individuals with low T, but did not differ between the fish that did or did not behave aggressively in the losing-experience training. Androgen receptor expression often is closely associated with aggression. Dominant male African cichlids (A. burtoni) express more AR mRNA in the anterior brain than subordinates [40]. In our study, only individuals assigned to losing experiences were included in this part of the analysis. Because AR gene expression in the fish was affected by contest experience, the effects from the losing experiences might have masked the difference (if any) in AR gene expression between aggressive and non-aggressive individuals.
(c). Relationships between pre-experience hormone levels and post-experience receptor gene expression levels
There was a positive correlation between the expression levels of all the receptor genes except AR, which had a strong negative relationship with 5-HT1AR expression. All receptor gene expression levels were positively correlated with T (AR non-significantly) but not F, despite the strong positive correlation between T and F.
Only AR and 5-HT1AR gene expression varied significantly with time-decay treatments: AR gene expression was lower while 5-HT1AR was higher at 0 h, suggesting that experimental procedures might have had opposite effects on them and caused the negative correlation. This hypothesis is supported by the fact that there was a negative correlation between AR and 5-HT1AR only at 0 h (r = −0.293, p = 0.003) and not at 3 h (r = −0.043, p = 0.671) or 48 h (r = 0.006, p = 0.957). This conclusion remains tentative, however, because restraint, chronic social stress and associated glucocorticoids potently downregulate 5-HT1AR, at least in the mammalian hippocampus [46,47]. Less is understood about the effects of stress on brain AR expression, but there is some evidence that early-life social instability attenuates it in the guinea pig hypothalamus [48]. Experimental treatment could have stimulated the sympathetic and/or neuroendocrine stress response circuits, reducing AR and elevating 5-HT1AR expression at 0 h. However, because baseline F correlated positively with AR expression and negatively (albeit non-significantly) with 5-HT1AR expression, perhaps baseline and stress-responsive neuroendocrine states produce different brain receptor expression patterns.
Various components of the hypothalamic–pituitary–adrenal and –gonadal axes and the serotonergic system regulate the activity of their own and the other systems [16,49]. For example, glucocorticoids and androgens exert opposite effects on brain 5-HT1AR [47], and oestrogens and androgens differentially affect HPA axis activity, although the direction of oestrogenic effects depends on whether ERα or ERβ transduce the signal [49]. Therefore, the pervasive correlations between receptor gene expression patterns, between F and T, and between hormones and receptor gene expression in this study are not surprising. It remains unclear why T was more strongly correlated with receptor expression than F. Our results indicate that, while baseline F and T are correlated, these hormones might operate on different neural circuits and perhaps drive different patterns of gene expression, as has been demonstrated in the complex, status-dependent networks that comprise sex steroid hormones and their receptors in cichlid fish [50]. Our data cannot speak to direct or indirect activation/inhibition of receptor expression by F or T (e.g. via ligand–receptor complexes acting as transcription factors, membrane receptor signal transduction cascades, upregulation of co-repressors or other cellular mediators). Characterization of promoter regions [51] coupled with genome-wide searches for glucocorticoid- and androgen-responsive genes [52,53] may help to resolve such causal relationships.
5. Conclusion
The difference in AR gene expression levels between fish given multiple wins and those given multiple losses in this study depended on baseline T. Winner–loser effects in this fish also depend on baseline T, strongly suggesting an important role for AR in driving experience-induced changes in contest performance. As T levels do not vary between individuals that have received different contest experiences [25], it appears that winner–loser effects operate by changing neural sensitivity to T rather than its titres. This study also showed that individuals with higher expression of ERα/β, 5-HT1AR and GR genes were more aggressive towards larger opponents, despite the fact that expression levels of these receptors did not vary between individuals receiving different contest experiences. The results of this study together with those of previous studies demonstrate that a combination of external (contest experience) and internal (baseline endocrine state and steroid hormone and serotonin receptor expression patterns) factors jointly modulate contest decisions in this fish.
Supplementary Material
Acknowledgements
We thank Alan Watson for help with comments and on the manuscript and Amanda Hanninen for assistance with hormone assays. We thank the two anonymous reviewers for thorough and helpful comments.
Ethics statement
This research was conducted in accordance with Institutional Animal Care and Use Committee at the University of Alabama (Protocol nos. 08-309 and 08-312).
Data accessibility
The dataset for this study has been deposited in the Dryad Repository (doi:10.5061/dryad.rr062). The DNA sequences of the five receptor genes used for this study are provided in the electronic supplementary material, S4.
Funding statement
This research was supported by Taiwan National Science Council (NSC 97-2621-B-003-005-MY3).
References
- 1.Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74. ( 10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 2.Oliveira RF, Silva A, Canário AVM. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. R. Soc. B 276, 2249–2256. ( 10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards DH, Kravitz EA. 1997. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 7, 812–819. ( 10.1016/S0959-4388(97)80140-7) [DOI] [PubMed] [Google Scholar]
- 4.Nelson RJ, Chiavegatto S. 2001. Molecular basis of aggression. Trends. Neurosci. 24, 713–719. ( 10.1016/S0166-2236(00)01996-2) [DOI] [PubMed] [Google Scholar]
- 5.Trainor BC, Kyomen HH, Marler CA. 2006. Estrogenic encounters: how interactions between aromatase and the environment modulate aggression. Front. Neuroendocrinol. 27, 170–179. ( 10.1016/j.yfrne.2005.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trainor BC, Bird IM, Marler CA. 2004. Opposing hormonal mechanisms of aggression revealed through short-lived testosterone manipulations and multiple winning experiences. Horm. Behav. 45, 115–121. ( 10.1016/j.yhbeh.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 7.Maxson SC. 2000. Genetic influences on aggressive behavior. In genetic influences on neural and behavioral functions (eds Pfaff DW. et al), pp. 405–416. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Simon NG, Gandelman R. 1978. The estrogenic arousal of aggressive behavior in female mice. Horm. Behav. 10, 118–127. ( 10.1016/0018-506X(78)90002-8) [DOI] [PubMed] [Google Scholar]
- 9.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. 1998. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl Acad. Sci. USA 94, 1476–1481. ( 10.1073/pnas.94.4.1476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa S, Chan J, Chester AE, Gustafsson J, Korach KS, Pfaff DW. 1999. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc. Natl Acad. Sci. USA 96, 12 887–12 892. ( 10.1073/pnas.96.22.12887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiBattista JD, Anisman H, Whitehead M, Gilmour KM. 2005. The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 208, 2707–2718. ( 10.1242/jeb.01690) [DOI] [PubMed] [Google Scholar]
- 12.Summers CH, Watt MJ, Ling TL, Forster GL, Carpenter RE, Korzan WJ, Lukkes JL, Øverli O. 2005. Glucocorticoid interaction with aggression in non-mammalian vertebrates: reciprocal action. Eur. J. Pharmacol. 526, 21–35. ( 10.1016/j.ejphar.2005.09.059) [DOI] [PubMed] [Google Scholar]
- 13.Kruk MR, Halász J, Meelis W, Haller J. 2004. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav. Neurosci. 118, 1062–1070. ( 10.1037/0735-7044.118.5.1062) [DOI] [PubMed] [Google Scholar]
- 14.Earley RL, Edwards JT, Aseem O, Felton K, Blumer LS, Karom M, Grober MS. 2006. Social interactions tune aggression and stress responsiveness in a territorial cichlid fish (Archocentrus nigrofasciatus). Physiol. Behav. 88, 353–363. ( 10.1016/j.physbeh.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 15.Clotfelter ED, O'Hare EP, McNitt MM, Carpenter RE, Summers CH. 2007. Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens. Pharmacol. Biochem. Behav. 87, 222–231. ( 10.1016/j.pbb.2007.04.018) [DOI] [PubMed] [Google Scholar]
- 16.Summers CH, Winberg S. 2006. Interactions between the neural regulation of stress and aggression. J. Exp. Biol. 209, 4581–4589. ( 10.1242/jeb.02565) [DOI] [PubMed] [Google Scholar]
- 17.Oyegbile TO, Marler CA. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267. ( 10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 18.Fuxjager MJ, Oyegbile TO, Marler CA. 2011. Independent and additive contributions of postvictory testosterone and social experience to the development of the winner effect. Endocrinology 152, 3422–3429. ( 10.1210/en.2011-1099) [DOI] [PubMed] [Google Scholar]
- 19.Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc. Natl. Acad. Sci. USA 107, 12 393–12 398. ( 10.1073/pnas.1001394107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschenhauser K, Gahr M, Goymann W. 2013. Winning and losing in public: audiences direct future success in Japanese quail. Horm. Behav. 63, 625–633. ( 10.1016/j.yhbeh.2013.02.010) [DOI] [PubMed] [Google Scholar]
- 21.Huang SP, Yang SY, Hsu Y. 2011. Persistence of winner and loser effects depends on the behaviour measured. Ethology 117, 171–180. ( 10.1111/j.1439-0310.2010.01856.x) [DOI] [Google Scholar]
- 22.Lan YT, Hsu Y. 2011. Prior dominance experience exerts a long-term influence on subsequent winner and loser effects. Front. Zool. 8, 28 ( 10.1186/1742-9994-8-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earley RL, Hsu Y. 2008. Reciprocity between endocrine state and contest behavior in the killifish, Kryptolebias marmoratus. Horm. Behav. 53, 442–451. ( 10.1016/j.yhbeh.2007.11.017) [DOI] [PubMed] [Google Scholar]
- 24.Chang C, Li CY, Earley RL, Hsu Y. 2012. Aggression and related behavioral traits: the impact of winning and losing and the role of hormones. Integr. Comp. Biol. 52, 801–813. ( 10.1093/icb/ics057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earley RL, Lu CK, Lee IH, Wong SC, Hsu Y. 2013. Winner and loser effects are modulated by hormonal states. Front. Zool. 10, 6 ( 10.1186/1742-9994-10-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor DS. 2012. Twenty-four years in the mud: what have we learned about the natural history and ecology of the mangrove rivulus, Kryptolebias marmoratus? Integr. Comp. Biol. 52, 724–736. ( 10.1093/icb/ics062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor DS. 1990. Adaptive specializations of the Cyprinodont fish Rivulus marmoratus. Fla. Sci. 53, 239–248. [Google Scholar]
- 28.Orlando EF, Katsu Y, Miyagawa S, Iguchi T. 2006. Cloning and differential expression of estrogen receptor and aromatase genes in the self-fertilizing hermaphrodite and male mangrove rivulus, Kryptolebias marmoratus. J. Mol. Endocrinol. 37, 353–365. ( 10.1677/jme.1.02101) [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. ( 10.1038/nprot.2008.73) [DOI] [PubMed] [Google Scholar]
- 30.Whitehouse MEA. 1997. Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 53, 913–923. ( 10.1006/anbe.1996.0313) [DOI] [Google Scholar]
- 31.Mesterton-Gibbons M. 1999. On the evolution of pure winner and loser effects: a game-theoretic model. Bull. Math. Biol. 61, 1151–1186. ( 10.1006/bulm.1999.0137) [DOI] [PubMed] [Google Scholar]
- 32.Parhar IS, Satoshi O, Hamada T, Sakuma Y. 2003. Single-cell real-time quantitative polymerase chain reaction of immunofluorescently identified neurons of gonadotropin-releasing hormone subtypes in cichlid fish. Endocrinology 144, 3297–3300. ( 10.1210/en.2003-0386) [DOI] [PubMed] [Google Scholar]
- 33.Santangelo N, Bass AH. 2006. New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc. R. Soc. B 273, 3085–3092. ( 10.1098/rspb.2006.3683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleszczyńska A, Sokołowska E, Kulczykowska E. 2012. Variation in brain arginine vasotocin (AVT) and isotocin (IT) levels with reproductive stage and social status in males of three-spined stickleback (Gasterosteus aculeatus). Gen. Comp. Endocrinol. 175, 290–296. ( 10.1016/j.ygcen.2011.11.022) [DOI] [PubMed] [Google Scholar]
- 35.Cooper MA, Karom M, Huhman KL, Albers HE. 2005. Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm. Behav. 48, 545–551. ( 10.1016/j.yhbeh.2005.04.012) [DOI] [PubMed] [Google Scholar]
- 36.Carpenter RE, Korzan WJ, Bockholt C, Watt MJ, Forster GL, Renner KJ, Summers CH. 2009. Corticotropin releasing factor influences aggression and monoamines: modulation of attacks and retreats. Neuroscience 158, 412–425. ( 10.1016/j.neuroscience.2008.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper MA, Huhman KL. 2005. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus). Behav. Neurosci. 119, 1042–1051. ( 10.1037/0735-7044.119.4.1042) [DOI] [PubMed] [Google Scholar]
- 38.Cooper MA, Huhman KL. 2007. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology 194, 297–307. ( 10.1007/s00213-007-0849-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. 2000. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (α/βERKO). Proc. Natl Acad. Sci. USA 97, 14 737–14 741. ( 10.1073/pnas.250473597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burmeister SS, Kailasanath V, Fernald RD. 2007. Social dominance regulates androgen and estrogen receptor gene expression. Horm. Behav. 51, 164–170. ( 10.1016/j.yhbeh.2006.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marini F, Pozzato C, Andreetta V, Jansson B, Arban R, Domenici E, Carboni L. 2006. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res. 1067, 25–35. ( 10.1016/j.brainres.2005.10.002) [DOI] [PubMed] [Google Scholar]
- 42.Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL. 2009. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European starling (Sturnus vulgaris) brain. J. Neuroendocrinol. 21, 832–840. ( 10.1111/j.1365-2826.2009.01908.x) [DOI] [PubMed] [Google Scholar]
- 43.Caramaschi D, de Boer SF, Koolhaas JM. 2007. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol. Behav. 90, 590–601. ( 10.1016/j.physbeh.2006.11.010) [DOI] [PubMed] [Google Scholar]
- 44.Korte SM, Meijer OC, de Kloet ER, Buwalda B, Keijser J, Sluyter F, van Oortmerssen G, Bohus B. 1996. Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res. 736, 338–343. ( 10.1016/0006-8993(96)00723-8) [DOI] [PubMed] [Google Scholar]
- 45.Cooper MA, Grober MS, Nicholas CR, Huhman KL. 2009. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience 161, 680–690. ( 10.1016/j.neuroscience.2009.03.084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmers DT, López JF, Vázquez DM, Akil H, Watson SJ. 1994. Regulation of hippocampal 5-HT1A receptor gene expression by dexamethasone. Neuropsychopharmacology 10, 215–222. ( 10.1038/npp.1994.24) [DOI] [PubMed] [Google Scholar]
- 47.Flügge G, Kramer M, Rensing S, Fuchs S. 1998. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur. J. Neurosci. 10, 2685–2693. ( 10.1046/j.1460-9568.1998.00280.x) [DOI] [PubMed] [Google Scholar]
- 48.Kaiser S, Sachser N. 2005. The effects of prenatal social stress on behaviour: mechanisms and function. Neurosci. Biobehav. Rev. 29, 283–294. ( 10.1016/j.neubiorev.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 49.Handa RJ, Weiser MJ. 2014. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 35, 197–220. ( 10.1016/j.yfrne.2013.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connell LA, Hofmann HA. 2012. Social status predicts how sex steroid receptors regulate complex behaviour across levels of biological organization. Endocrinology 153, 1341–1351. ( 10.1210/en.2011-1663) [DOI] [PubMed] [Google Scholar]
- 51.Falkenberg VR, Rajeevan MS. 2010. Identification of a potential molecular link between the glucocorticoid and serotonergic signaling systems. J. Mol. Neurosci. 41, 322–327. ( 10.1007/s12031-009-9320-6) [DOI] [PubMed] [Google Scholar]
- 52.Le PP, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH. 2005. Glucocorticoid receptor-dependent gene regulatory networks. PLoS. Genet. 1, e16 ( 10.1371/journal.pgen.0010016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang M, et al. 2009. Androgen-responsive gene database: integrated knowledge of androgen-responsive genes. Mol. Endocrinol. 23, 1927–1933. ( 10.1210/me.2009-0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset for this study has been deposited in the Dryad Repository (doi:10.5061/dryad.rr062). The DNA sequences of the five receptor genes used for this study are provided in the electronic supplementary material, S4.


