Abstract
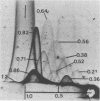
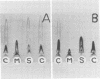
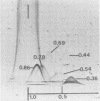
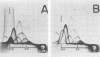
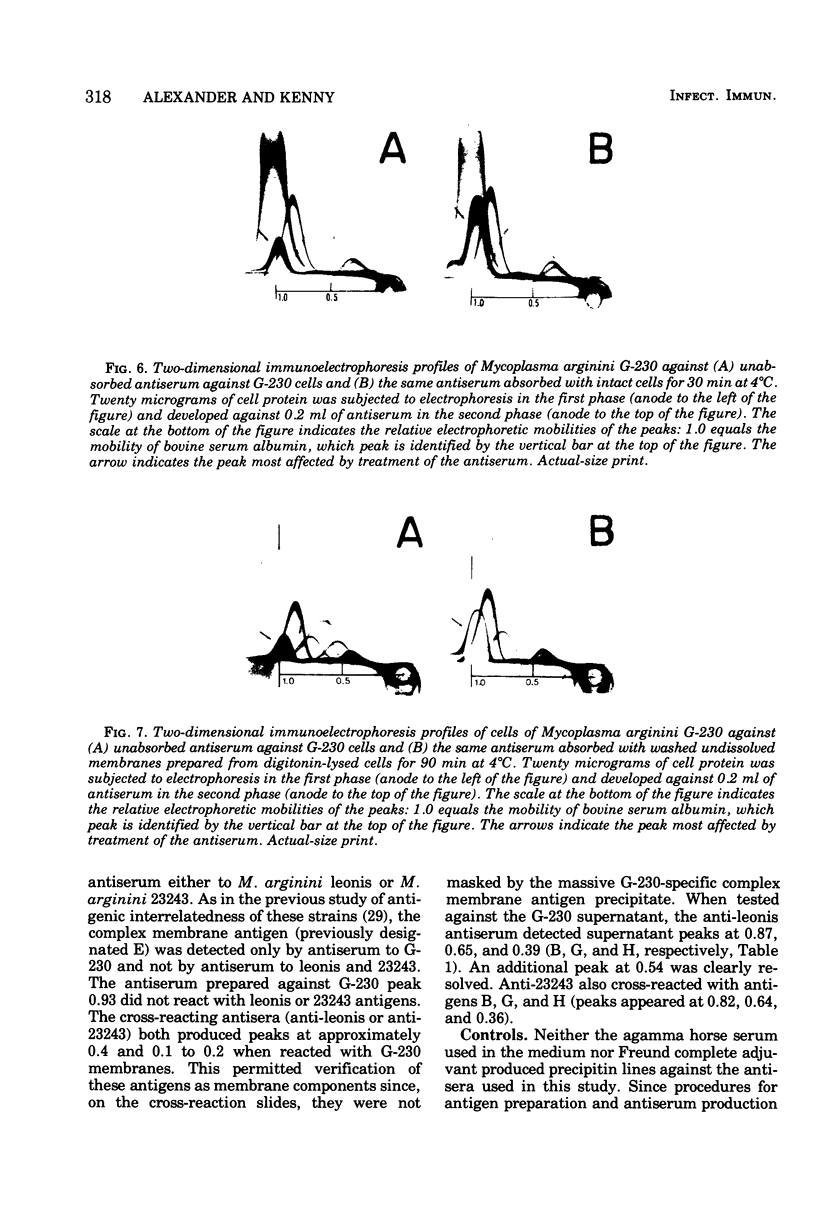
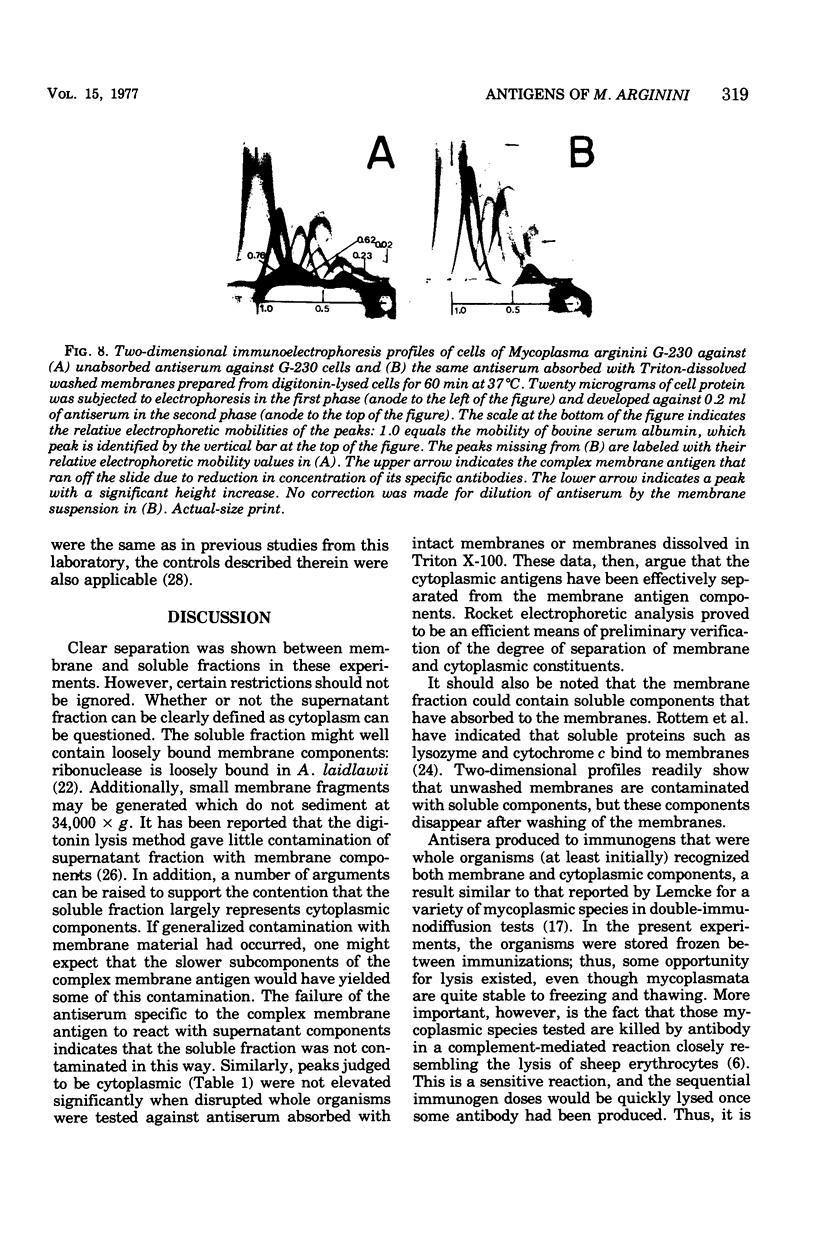
Two-dimensional immunoelectrophoresis was employed to electrophoretically identify membrane and cytoplasmic antigens of Mycoplasma arginini G-230. Five distinct cytoplasmic antigens were observed in soluble fractions prepared by digitonin lysis with electrophoretic mobilities (relative to bovine albumin) ranging from 0.36 to 0.86; four of these were common to other M. arginini strains: leonis and 23243. Five membrane antigens were identified, two of which (0.4 and 0.2) were common to the other M. arginini strains. The most prominent antigenic component of the membrane fraction (the complex membrane antigen) was electrophoretically heterogeneous, showing four antigenically related components with electrophoretic mobilities of 1.2, 0.95 to 0.76 and 0.05. The complex membrane antigen was exposed on the outside of the mycoplasmic cell because absorption of antiserum with live organisms removed antibody to this component. Antibodies to two other membrane components (0.6 and 0.2) were removed by absorption with Triton-solubilized membranes, but not by untreated membranes, indicating that these components were, at best, little exposed on either membrane surface. Antiserum was prepared against the complex membrane antigen using precipitin lines from two dimensional electropherograms as the immunogen. This antiserum reacted only with the complex membrane antigen and did not react with the other M. arginini strains, indicating that the complex membrane antigen was unique to strain G-230.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar A., Rottem S., Razin S. Characterization of the mycoplasma membrane proteins. IV. Disposition of proteins in the membrane. Biochim Biophys Acta. 1974 Jun 13;352(2):228–244. doi: 10.1016/0005-2736(74)90214-4. [DOI] [PubMed] [Google Scholar]
- Argaman M., Razin S. Antigenic properties of mycoplasma organisms and membranes. J Gen Microbiol. 1969 Jan;55(1):45–58. doi: 10.1099/00221287-55-1-45. [DOI] [PubMed] [Google Scholar]
- Barile M. F., DelGiudice R. A., Carski T. R., Gibbs C. J., Morris J. A. Isolation and characterization of Mycoplasma arginini: spec. nov. Proc Soc Exp Biol Med. 1968 Nov;129(2):489–494. doi: 10.3181/00379727-129-33351. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Revis G. J., Jarrett K. Preparatory electroimmunodiffusion for making precipitins to selected native antigens. Immunol Commun. 1972;1(4):325–336. doi: 10.3109/08820137209022946. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gale J. L., Kenny G. E. Complement dependent killing of Mycoplasma pneumoniae by antibody: kinetics of the reaction. J Immunol. 1970 May;104(5):1175–1183. [PubMed] [Google Scholar]
- Hahn R. G., Kenny G. E. Differences in arginine requirement for growth among arginine-utilizing Mycoplasma species. J Bacteriol. 1974 Feb;117(2):611–618. doi: 10.1128/jb.117.2.611-618.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Antigenic differences within the species Mycoplasma hominis. J Hyg (Lond) 1970 Sep;68(3):469–477. doi: 10.1017/s0022172400042376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Membrane antigens of Mycoplasma hominis. J Hyg (Lond) 1972 Mar;70(1):85–98. doi: 10.1017/s0022172400022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K. E., Hjertén S. Localization of the Tween 20-soluble membrane proteins of Acholeplasma laidlawii by crossed immunoelectrophoresis. J Mol Biol. 1974 Jun 25;86(2):341–348. doi: 10.1016/0022-2836(74)90023-0. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Immunological analysis of Mycoplasma membranes. J Bacteriol. 1969 Oct;100(1):187–194. doi: 10.1128/jb.100.1.187-194.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Immunogenicity of Mycoplasma pneumoniae. Infect Immun. 1971 Apr;3(4):510–515. doi: 10.1128/iai.3.4.510-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. On the immunoelectrophoretical identification and quantitation of serum proteins. Scand J Clin Lab Invest. 1968;22(1):79–81. doi: 10.3109/00365516809160742. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levisohn S., Razin S. Isolation, ultrastructure and antigenicity of Mycoplasma gallisepticum membranes. J Hyg (Lond) 1973 Dec;71(4):725–737. doi: 10.1017/s0022172400022981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman Z., Kahane I., Kovartovsky J., Razin S. Characterization of the myoplasma membrane proteins. 3. Gel filtration and immunological characterization of Acholeplasma laidlawii membrane proteins. Biochim Biophys Acta. 1972 Apr 14;266(1):255–268. doi: 10.1016/0005-2736(72)90140-x. [DOI] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. Binding of proteins to mycoplasma membranes. Biochim Biophys Acta. 1973 Apr 16;298(4):876–886. doi: 10.1016/0005-2736(73)90392-1. [DOI] [PubMed] [Google Scholar]
- Rottem S. Heterogeneity in the physical state of the exterior and interior regions of Mycoplasma membrane lipids. Biochem Biophys Res Commun. 1975 May 5;64(1):7–12. doi: 10.1016/0006-291x(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Thirkill C. E., Kenny G. E. Antigenic analysis of three strains of Mycoplasma arginini by two-dimensional immunoelectrophoresis. J Immunol. 1975 Mar;114(3):1107–1111. [PubMed] [Google Scholar]
- Thirkill C. E., Kenny G. E. Serological comparison of five arginine-utilizing Mycoplasma species by two-dimensional immunoelectrophoresis. Infect Immun. 1974 Sep;10(3):624–632. doi: 10.1128/iai.10.3.624-632.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]