Abstract
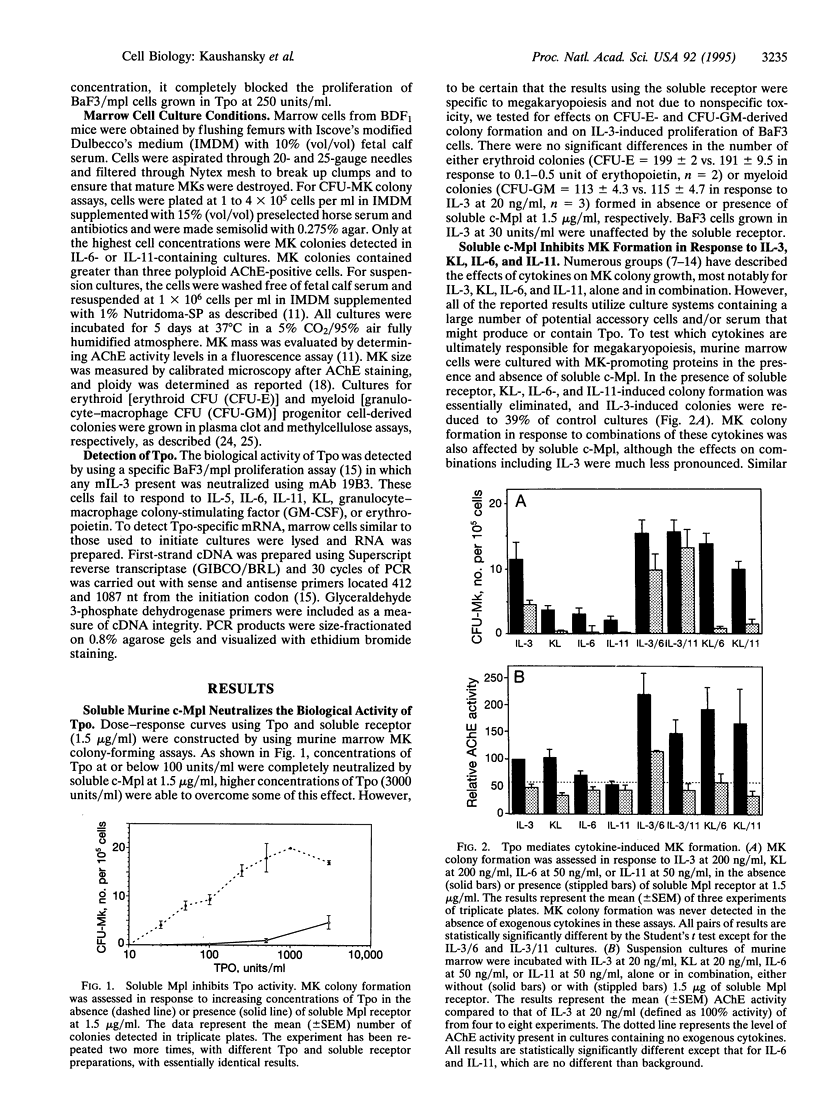
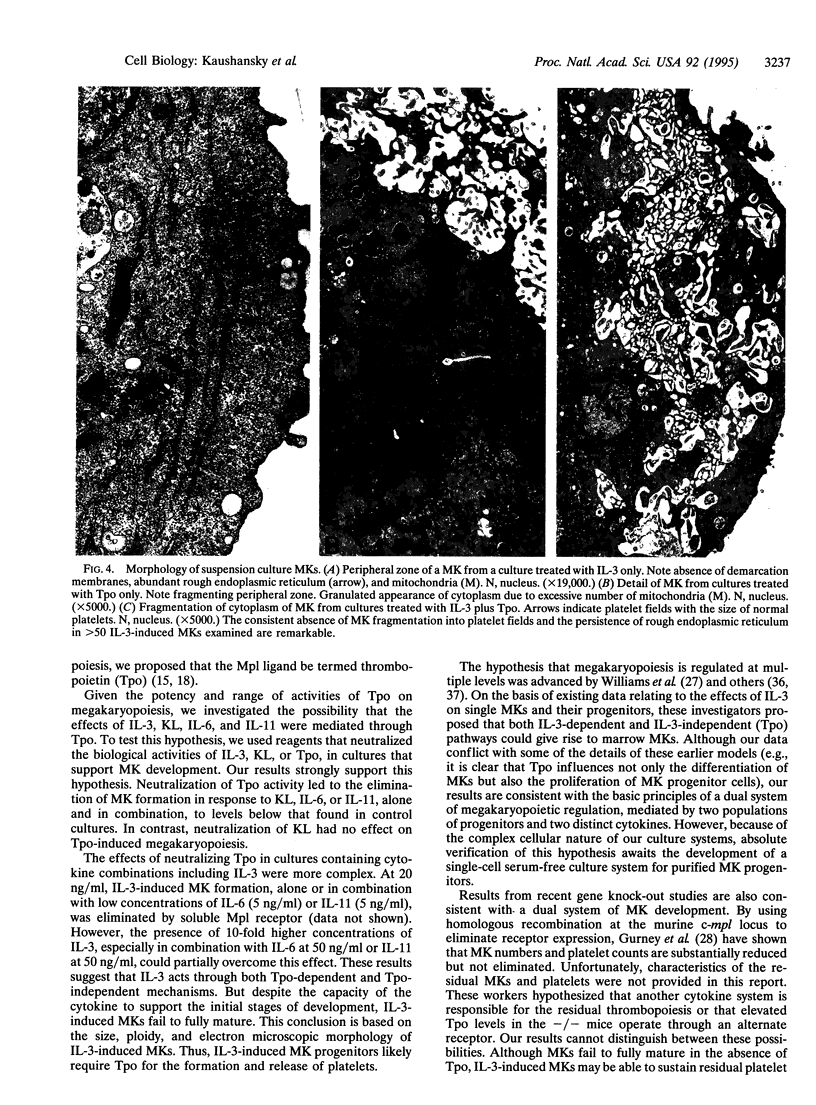
The development of megakaryocytes (MKs) from their marrow precursors is one of the least understood aspects of hematopoiesis. Current models suggest that early-acting MK colony-stimulating factors, such as interleukin (IL) 3 or c-kit ligand, are required for expansion of hematopoietic progenitors into cells capable of responding to late-acting MK potentiators, including IL-6 and IL-11. Recently, the Mp1 ligand, or thrombopoietin (Tpo), has been shown to display both MK colony-stimulating factor and potentiator activities, at potencies far greater than that of other cytokines. In light of these findings, we tested the hypothesis that Tpo is absolutely necessary for MK development. In this report we demonstrate that neutralizing the biological activity of Tpo eliminates MK formation in response to c-kit ligand, IL-6, and IL-11, alone and in combination, but that these reagents only partially reduce MK formation in the presence of combinations of cytokines including IL-3. However, despite the capacity of IL-3 to support the proliferation and initial stages of MK differentiation, elimination of Tpo prevents the full maturation of IL-3-induced MK. These data indicate that two populations of MK progenitors can be identified: one that is responsive to IL-3 but can fully develop only in the presence of Tpo and a second that is dependent on Tpo for both proliferation and differentiation. Thus, our results strongly suggest that Tpo is the primary regulator of MK development and platelet production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham H., Vannier E., Cowley S., Jiang S. X., Chi S., Dinarello C. A., Zsebo K. M., Groopman J. E. Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood. 1992 Jan 15;79(2):365–371. [PubMed] [Google Scholar]
- Bartley T. D., Bogenberger J., Hunt P., Li Y. S., Lu H. S., Martin F., Chang M. S., Samal B., Nichol J. L., Swift S. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994 Jul 1;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Brandt J. E., Bhalla K., Hoffman R. Effects of interleukin-3 and c-kit ligand on the survival of various classes of human hematopoietic progenitor cells. Blood. 1994 Mar 15;83(6):1507–1514. [PubMed] [Google Scholar]
- Briddell R. A., Brandt J. E., Leemhuis T. B., Hoffman R. Role of cytokines in sustaining long-term human megakaryocytopoiesis in vitro. Blood. 1992 Jan 15;79(2):332–337. [PubMed] [Google Scholar]
- Briddell R. A., Bruno E., Cooper R. J., Brandt J. E., Hoffman R. Effect of c-kit ligand on in vitro human megakaryocytopoiesis. Blood. 1991 Dec 1;78(11):2854–2859. [PubMed] [Google Scholar]
- Broudy V. C., Tait J. F., Powell J. S. Recombinant human erythropoietin: purification and analysis of carbohydrate linkage. Arch Biochem Biophys. 1988 Sep;265(2):329–336. doi: 10.1016/0003-9861(88)90135-x. [DOI] [PubMed] [Google Scholar]
- Burstein S. A., Mei R. L., Henthorn J., Friese P., Turner K. Leukemia inhibitory factor and interleukin-11 promote maturation of murine and human megakaryocytes in vitro. J Cell Physiol. 1992 Nov;153(2):305–312. doi: 10.1002/jcp.1041530210. [DOI] [PubMed] [Google Scholar]
- Dai C. H., Krantz S. B., Zsebo K. M. Human burst-forming units-erythroid need direct interaction with stem cell factor for further development. Blood. 1991 Nov 15;78(10):2493–2497. [PubMed] [Google Scholar]
- De Gabriele G., Penington D. G. Physiology of the regulation of platelet production. Br J Haematol. 1967 Mar;13(2):202–209. doi: 10.1111/j.1365-2141.1967.tb08732.x. [DOI] [PubMed] [Google Scholar]
- Debili N., Massé J. M., Katz A., Guichard J., Breton-Gorius J., Vainchenker W. Effects of the recombinant hematopoietic growth factors interleukin-3, interleukin-6, stem cell factor, and leukemia inhibitory factor on the megakaryocytic differentiation of CD34+ cells. Blood. 1993 Jul 1;82(1):84–95. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Wang E. A., Wong G. G., Clark S. C., Nathan D. G. Purification of fetal hematopoietic progenitors and demonstration of recombinant multipotential colony-stimulating activity. J Clin Invest. 1985 Sep;76(3):1286–1290. doi: 10.1172/JCI112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. L., Carver-Moore K., de Sauvage F. J., Moore M. W. Thrombocytopenia in c-mpl-deficient mice. Science. 1994 Sep 2;265(5177):1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- Heard J. M., Fichelson S., Varet B. Role of colony-stimulating activity in murine long-term bone marrow cultures: evidence for its production and consumption by the adherent cells. Blood. 1982 Apr;59(4):761–767. [PubMed] [Google Scholar]
- Hill R. J., Levin J., Levin F. C. Correlation of in vitro and in vivo biological activities during the partial purification of thrombopoietin. Exp Hematol. 1992 Mar;20(3):354–360. [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Uchida T., Kariyone S., Friese P., Burstein S. A. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Lok S., Holly R. D., Broudy V. C., Lin N., Bailey M. C., Forstrom J. W., Buddle M. M., Oort P. J., Hagen F. S. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994 Jun 16;369(6481):568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Berkner K., Segal G. M., Hagen F. S., Adamson J. W. Genomic cloning, characterization, and multilineage growth-promoting activity of human granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3101–3105. doi: 10.1073/pnas.83.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., O'Hara P. J., Hart C. E., Forstrom J. W., Hagen F. S. Role of carbohydrate in the function of human granulocyte-macrophage colony-stimulating factor. Biochemistry. 1987 Jul 28;26(15):4861–4867. doi: 10.1021/bi00389a038. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. The molecular mechanism of erythropoietin action. Eur J Biochem. 1992 Dec 15;210(3):649–663. doi: 10.1111/j.1432-1033.1992.tb17466.x. [DOI] [PubMed] [Google Scholar]
- Li B. L., Langer J. A., Schwartz B., Pestka S. Creation of phosphorylation sites in proteins: construction of a phosphorylatable human interferon alpha. Proc Natl Acad Sci U S A. 1989 Jan;86(2):558–562. doi: 10.1073/pnas.86.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Kaushansky K., Holly R. D., Kuijper J. L., Lofton-Day C. E., Oort P. J., Grant F. J., Heipel M. D., Burkhead S. K., Kramer J. M. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994 Jun 16;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- Long M. W., Hutchinson R. J., Gragowski L. L., Heffner C. H., Emerson S. G. Synergistic regulation of human megakaryocyte development. J Clin Invest. 1988 Nov;82(5):1779–1786. doi: 10.1172/JCI113791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. P. Thrombopoietin: its biology, purification, and characterization. Exp Hematol. 1988 Mar;16(3):201–205. [PubMed] [Google Scholar]
- McNiece I., Andrews R., Stewart M., Clark S., Boone T., Quesenberry P. Action of interleukin-3, G-CSF, and GM-CSF on highly enriched human hematopoietic progenitor cells: synergistic interaction of GM-CSF plus G-CSF. Blood. 1989 Jul;74(1):110–114. [PubMed] [Google Scholar]
- Messner H. A., Yamasaki K., Jamal N., Minden M. M., Yang Y. C., Wong G. G., Clark S. C. Growth of human hemopoietic colonies in response to recombinant gibbon interleukin 3: comparison with human recombinant granulocyte and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6765–6769. doi: 10.1073/pnas.84.19.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Hemopoietic regulators. Trends Biochem Sci. 1992 Aug;17(8):286–289. doi: 10.1016/0968-0004(92)90436-d. [DOI] [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Paul C. C., Tolbert M., Mahrer S., Singh A., Grace M. J., Baumann M. A. Cooperative effects of interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor: a new myeloid cell line inducible to eosinophils. Blood. 1993 Mar 1;81(5):1193–1199. [PubMed] [Google Scholar]
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M., Jr, Leary A. C., Sibley B., Clark S. C., Williams D. A. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P. J., McGrath H. E., Williams M. E., Robinson B. E., Deacon D. H., Clark S., Urdal D., McNiece I. K. Multifactor stimulation of megakaryocytopoiesis: effects of interleukin 6. Exp Hematol. 1991 Jan;19(1):35–41. [PubMed] [Google Scholar]
- Segal G. M., Stueve T., Adamson J. W. Analysis of murine megakaryocyte colony size and ploidy: effects of interleukin-3. J Cell Physiol. 1988 Dec;137(3):537–544. doi: 10.1002/jcp.1041370320. [DOI] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Greenberger J. S., Dexter T. M. Production of colony stimulating factor in long-term bone marrow cultures. J Cell Physiol. 1983 Jan;114(1):88–92. doi: 10.1002/jcp.1041140115. [DOI] [PubMed] [Google Scholar]
- Wendling F., Maraskovsky E., Debili N., Florindo C., Teepe M., Titeux M., Methia N., Breton-Gorius J., Cosman D., Vainchenker W. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994 Jun 16;369(6481):571–574. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Walker F., Oon S. H. Multiple levels of regulation of megakaryocytopoiesis. Blood Cells. 1989;15(1):123–133. [PubMed] [Google Scholar]
- de Sauvage F. J., Hass P. E., Spencer S. D., Malloy B. E., Gurney A. L., Spencer S. A., Darbonne W. C., Henzel W. J., Wong S. C., Kuang W. J. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994 Jun 16;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]




