Abstract
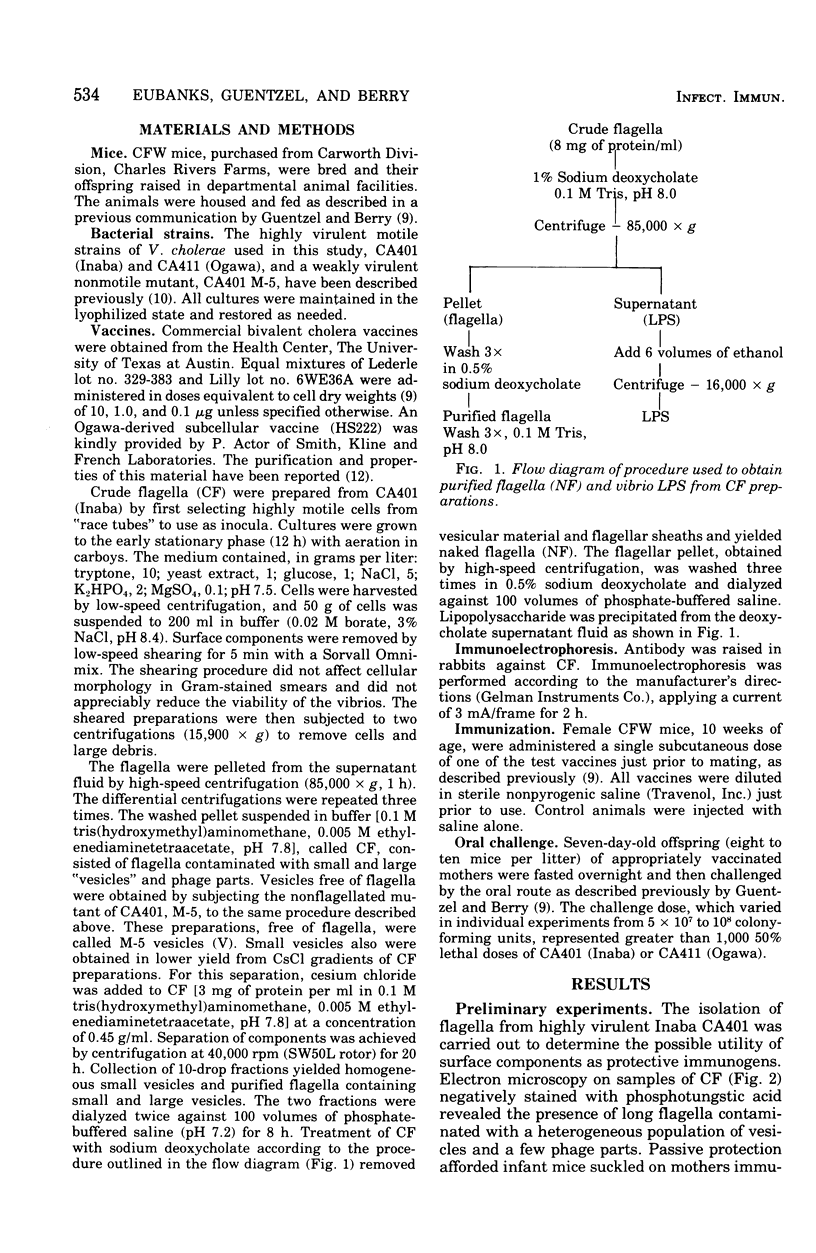
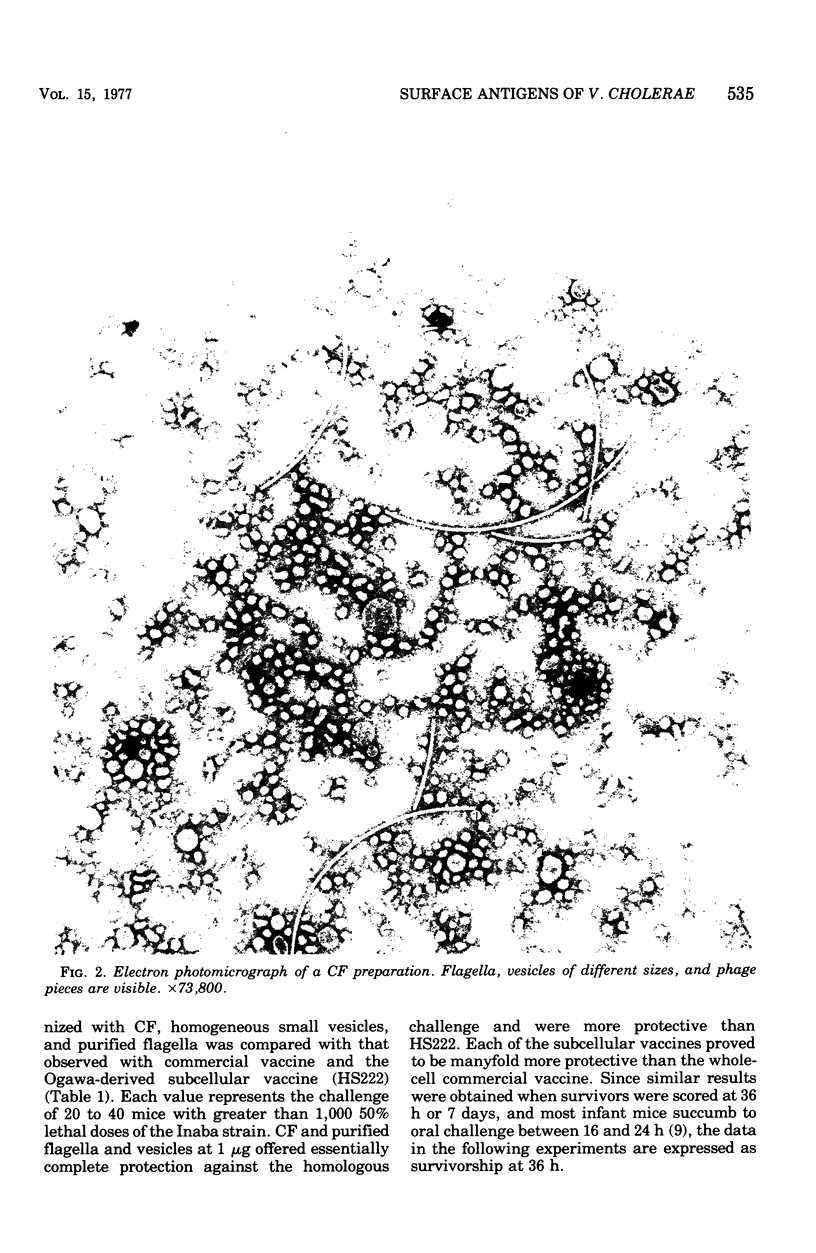
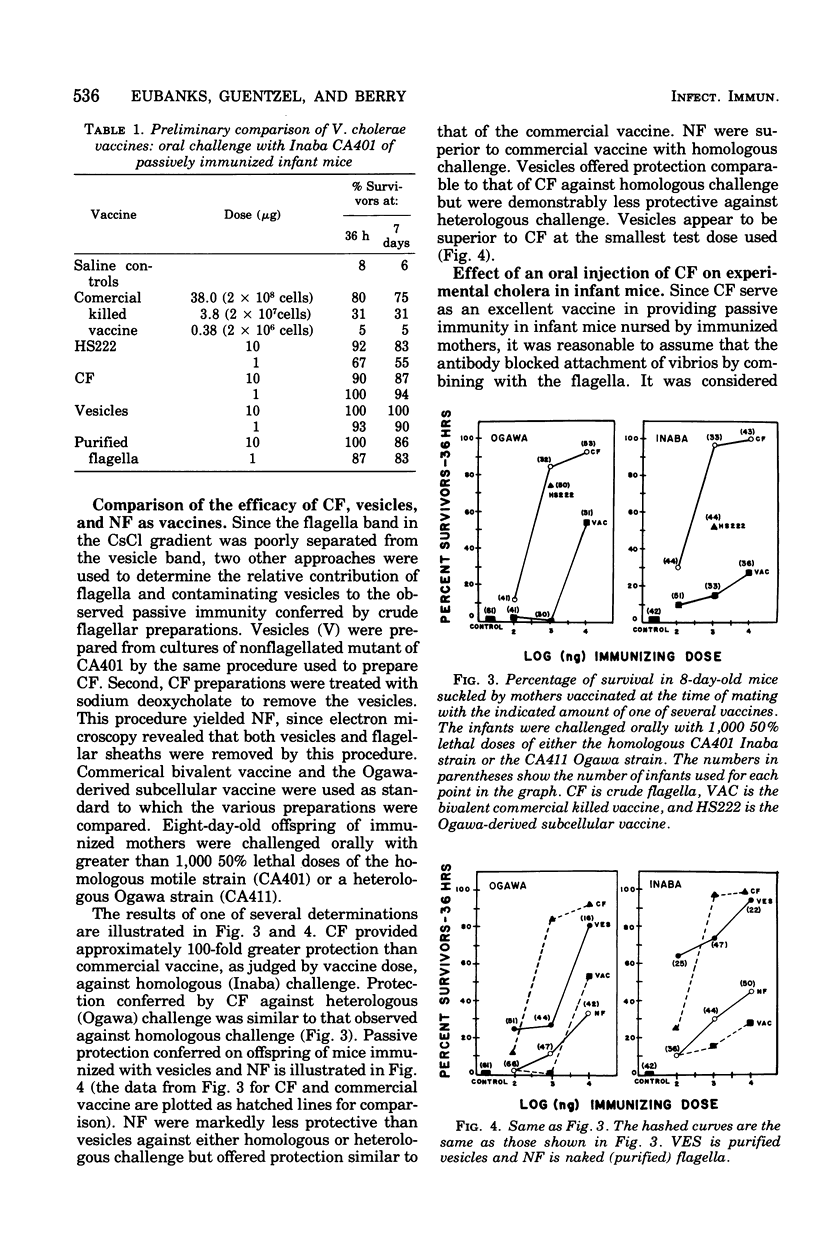
Surface components of a motile Inaba strain (CA401) were removed from washed cells by low-speed shearing. Flagella contaminated with a vesicular material (designated as crude flagella [CFA1) were obtained by differential centrifugation of the shear fluid. Vesicles were obtained from a nonflagellated mutant by the same procedure. Homogeneous small vesicles were obtained in diminished yield from CsCl gradients of CF preparations. Treatment of CF with sodium deoxycholate removed the vesicular material and flagellar sheaths and yielded naked flagella (NF). The ability of these preparations of passively protect infant mice suckled by CFW mothers that had been immunized at the time of mating was compared, on a dry-weight basis, with commercial vaccine (CV). Eight-day-old mice were challenged orally with more than 1,000 50% lethal doses of either the homologous or a heterologous (Ogawa Ca411) strain. The most effective immunogen was CF, which provided complete protection at 1 microng against both challenges. CF and vesicles provided 50- to 100-fold greater protection than CV against homologous challenge. With heterologous challenge, vesicles were 10-fold more protective than CV, markedly less protective than CF. The NF offered only slightly greater protection than CV against both challenges. Immunoelectrophoresis revealed an antigen in CF distinct from vesicles, cell wall lipopolysaccharide or NF. This antigen is not present in the nonflagellated mutant and is apparently associated with motility,
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENENSON A. S., ISLAM M. R., GREENOUGH W. B., 3rd RAPID IDENTIFICATION OF VIBRIO CHOLERAE BY DARKFIELD MICROSCOPY. Bull World Health Organ. 1964;30:827–831. [PMC free article] [PubMed] [Google Scholar]
- Bellamy J. E., Knop J., Steele E. J., Chaicumpa W., Rowley D. Antibody cross-linking as a factor in immunity to cholera in infant mice. J Infect Dis. 1975 Aug;132(2):181–188. doi: 10.1093/infdis/132.2.181. [DOI] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Virulence factors involved in the intraperitoneal infection of adult mice with Vibrio cholerae. Infect Immun. 1976 Feb;13(2):457–463. doi: 10.1128/iai.13.2.457-463.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT E. A., GORDON J. AN ELECTRON MICROSCOPE STUDY OF VIBRIO FLAGELLA. J Gen Microbiol. 1963 Aug;32:235–239. doi: 10.1099/00221287-32-2-235. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A. Immunology of cholera. Curr Top Microbiol Immunol. 1975;69:138–196. [PubMed] [Google Scholar]
- Freter R. Mechanism of Action of Intestinal Antibody in Experimental Cholera II. Antibody-Mediated Antibacterial Reaction at the Mucosal Surface. Infect Immun. 1970 Nov;2(5):556–562. doi: 10.1128/iai.2.5.556-562.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Parameters affecting the association of vibrios with the intestinal surface in experimental cholera. Infect Immun. 1972 Aug;6(2):134–141. doi: 10.1128/iai.6.2.134-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Protection of suckling mice from experimental cholera by maternal immunization: comparison of the efficacy of whole-cell, ribosomal-derived, and enterotoxin immunogens. Infect Immun. 1974 Jul;10(1):167–172. doi: 10.1128/iai.10.1.167-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Field L. H., Eubanks E. R., Berry L. J. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun. 1977 Feb;15(2):539–548. doi: 10.1128/iai.15.2.539-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Gregory B., Naylor J., Actor P. Isolation of protective somatic antigen from Vibrio cholerae (Ogawa) ribosomal preparations. Infect Immun. 1972 Aug;6(2):156–161. doi: 10.1128/iai.6.2.156-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANKFORD C. E. Factors of virulence of Vibrio cholerae. Ann N Y Acad Sci. 1960 Nov 21;88:1203–1212. doi: 10.1111/j.1749-6632.1960.tb20111.x. [DOI] [PubMed] [Google Scholar]
- Pitkin D., Actor P. Immunity to Vibrio cholerae in the mouse. I. Passive protection of newborn mice. Infect Immun. 1972 Apr;5(4):428–432. doi: 10.1128/iai.5.4.428-432.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele E. J., Chaicumpa W., Rowley D. Further evidence for cross-linking as a protective factor in experimental cholera: properties of antibody fragments. J Infect Dis. 1975 Aug;132(2):175–180. doi: 10.1093/infdis/132.2.175. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Yalow R., Berson S. A. Detection of Australia antigen and antibody by means of radioimmunoassay techniques. J Infect Dis. 1970 May;121(5):550–554. doi: 10.1093/infdis/121.5.550. [DOI] [PubMed] [Google Scholar]



