Abstract
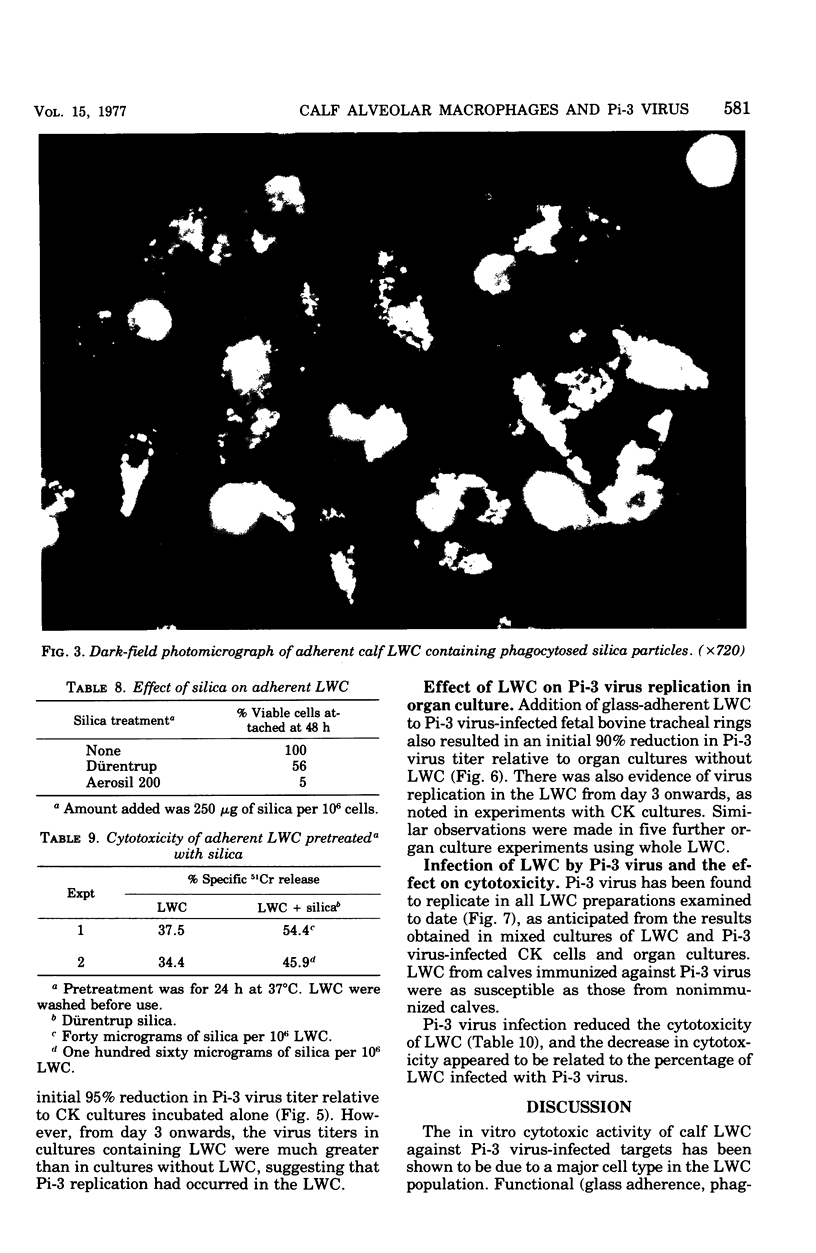
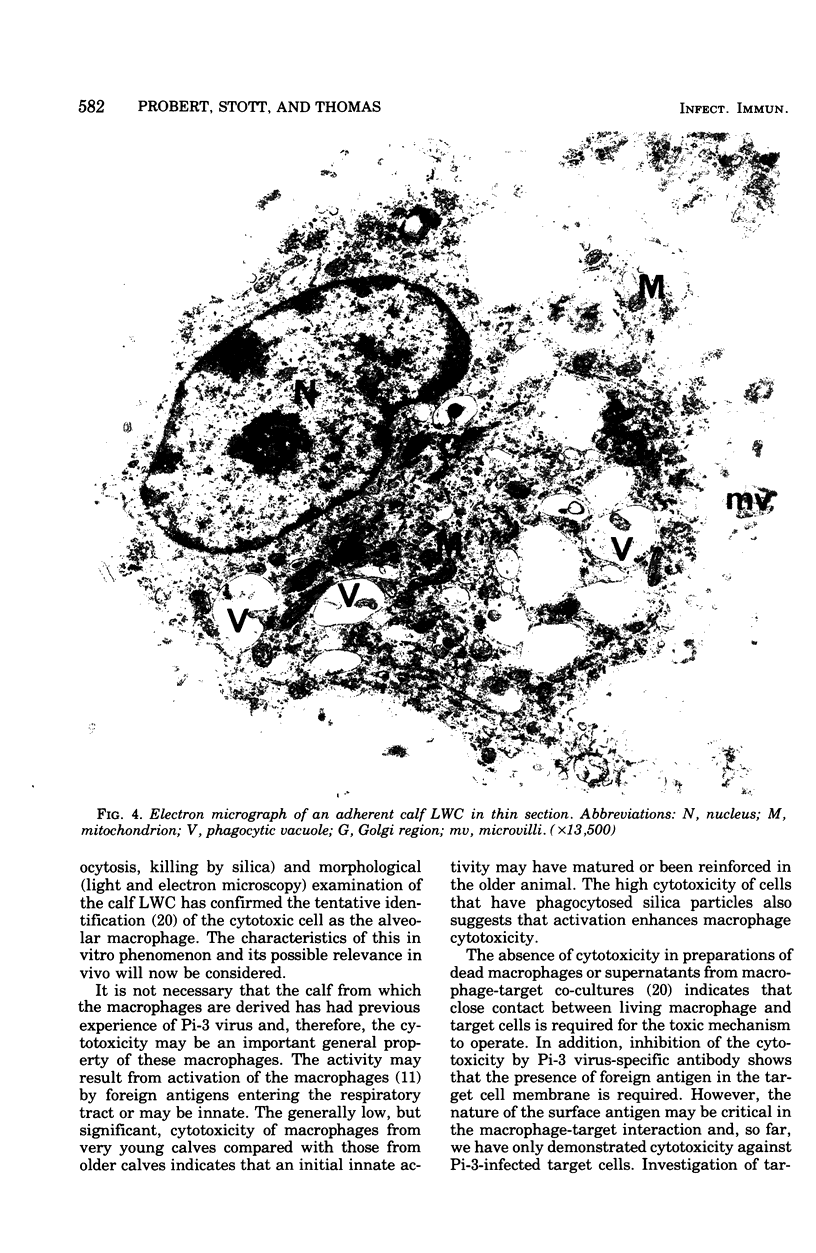
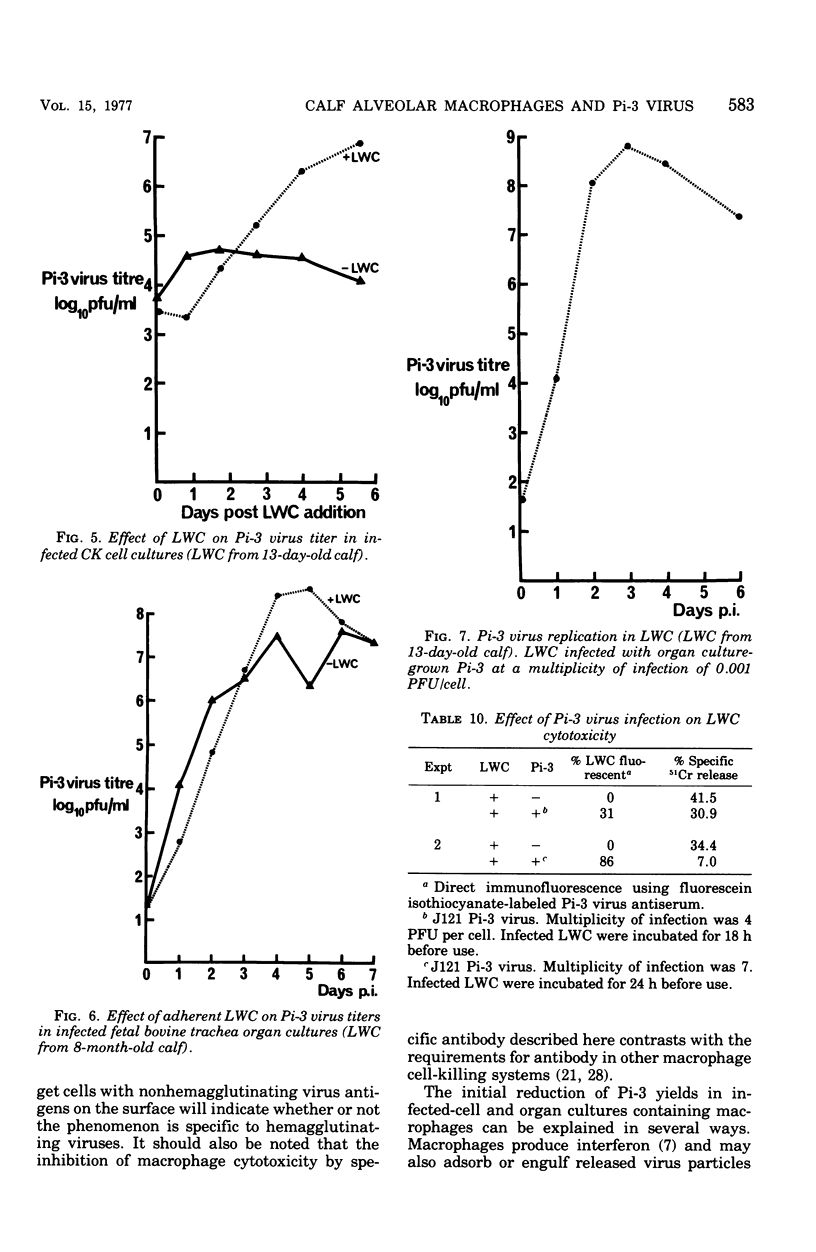
Cells washed from the lungs of freshly killed calves (lung wash cells; LWC) were cytotoxic for calf kidney (CK) target cells infected with parainfluenzavirus type 3 (Pi-3) when assayed by chromium release. LWC collected from 25 calves, including two gnotobiotic animals that had not previously been infected with Pi-3, were all cytotoxic, giving a specific chromium release between 11 and 50%. Cytotoxicity was detected at ratios of LWC to target cell as low as 5:1. The cytotoxic reaction required viable LWC, was inhibited by Pi-3 antiserum, and was not the result of virus-induced damage to the target cells. The cytotoxic cells in the LWC population were identified as alveolar macrophages from observations on glass adherence, phagocytic activity, killing by silica and fine-structural appearance. When LWC were added to CK cells or organ cultures of bovine trachea infected with Pi-3, the yield of virus was reduced for the first 2 to 3 days. However, subsequently, Pi-3 virus replicated in the LWC. Infection of LWC with Pi-3 virus reduced their cytotoxic activity. The significance of these interactions between alveolar macrophages and Pi-3 virus is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Avila F. R., Schultz R. M., Tompkins W. A. Specific macrophage immunity to vaccinia virus: macrophage-virus interaction. Infect Immun. 1972 Jul;6(1):9–16. doi: 10.1128/iai.6.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda R., Danes L., Cinátl J. Infection of rabbit alveolar macrophages with a neurotropic vaccinia virus strain in vitro. Acta Virol. 1970 Sep;14(5):353–361. [PubMed] [Google Scholar]
- Bey E., Harington J. S. Cytotoxic effects of some mineral dusts on Syrian hamster peritoneal macrophages. J Exp Med. 1971 May 1;133(5):1149–1169. doi: 10.1084/jem.133.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASGOW L. A. LEUKOCYTES AND INTERFERON IN THE HOST RESPONSE TO VIRAL INFECTIONS. I. MOUSE LEUKOCYTES AND LEUKOCYTE-PRODUCED INTERFERON IN VACCINIA VIRUS INFECTION IN VITRO. J Exp Med. 1965 Jun 1;121:1001–1018. doi: 10.1084/jem.121.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Kruse F., Thomssen R. Interactions between vaccinia virus and sensitized macrophages in vitro. Arch Virol. 1975;48(4):335–345. doi: 10.1007/BF01317432. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther P. D. Preparation of monolayer pig kidney cell cultures by a modified continuous flow trypsinization procedure. Med Lab Technol. 1972 Apr;29(2):191–194. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K. Establisment of the AU-BEK cell line and comparison with two other bovine cell lines. In Vitro. 1973 Nov-Dec;9(3):147–155. doi: 10.1007/BF02618431. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Defense mechanisms against infectious bovine rhinotracheitis virus: inhibition of virus infection by murine macrophages. Infect Immun. 1975 Mar;11(3):505–511. doi: 10.1128/iai.11.3.505-511.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicka K., Daly B. D., Migliore J. J., Norman J. C. Ultrastructure of pulmonary alveoli of the calf. Am J Vet Res. 1974 Feb;35(2):213–222. [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Stott E. J., Probert M., Thomas L. H. Cytotoxicity of alveolar macrophages for virus-infected cells. Nature. 1975 Jun 26;255(5511):710–712. doi: 10.1038/255710a0. [DOI] [PubMed] [Google Scholar]
- Temple A., Loewi G., Davies P., Howard A. Cytotoxicity of immune guinea-pig cells. II. The mechanism of macrophage cytotoxicity. Immunology. 1973 Apr;24(4):655–669. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Howard C. J. Effect of Mycoplasma dispar, Mycoplasma bovirhinis, Acholeplasma laidlawii and T-mycoplasmas on explant cultures of bovine trachea. J Comp Pathol. 1974 Apr;84(2):193–201. doi: 10.1016/0021-9975(74)90060-7. [DOI] [PubMed] [Google Scholar]
- Tompkins W. A., Zarling J. M., Rawls W. E. In vitro assessment of cellular immunity to vaccinia virus: contribution of lymphocytes and macrophages. Infect Immun. 1970 Dec;2(6):783–790. doi: 10.1128/iai.2.6.783-790.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Nozima T. Delayed hypersensitivity in vaccinia-infected mice. II. Resistance of peritoneal macrophages against vaccinia infection. Acta Virol. 1973 Jan;17(1):41–49. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. Immunity to infections on secretory surfaces. J Infect Dis. 1974 Oct;130(4):419–440. doi: 10.1093/infdis/130.4.419. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Weissman I., Lannin D., Jerabek L., Berclay T. Cellular immunity to heterologous erythrocytes in vitro. I. The role of surface-adherent cells and specific mediators in an effector mechanism. Cell Immunol. 1973 May;7(2):222–236. doi: 10.1016/0008-8749(73)90245-1. [DOI] [PubMed] [Google Scholar]