Abstract
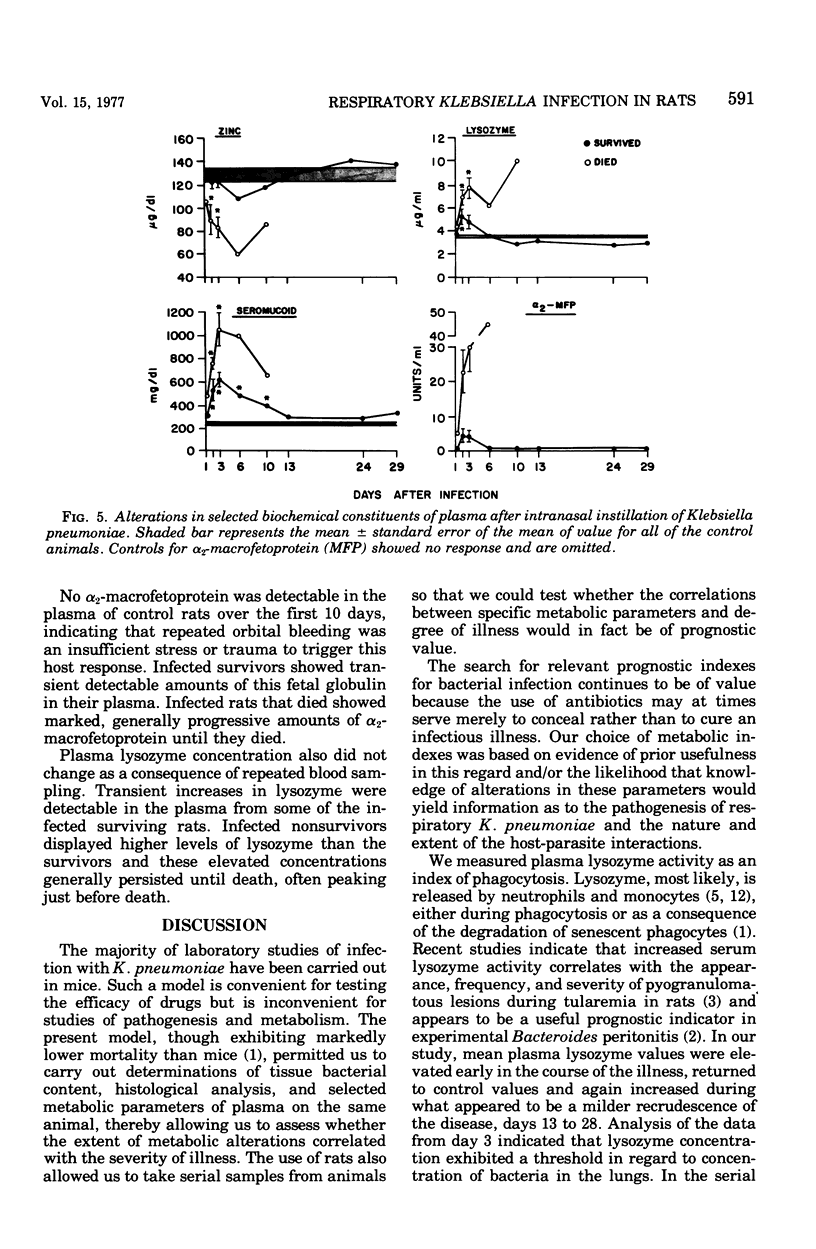
Gram-negative bacterial pneumonias have been increasingly important as nosocomial infections. The following model was developed to study the pathogenesis and evaluate therapy of such infections. Intranasal instillation of rats with a suspension of 5 x 10(6) Klebsiella pneumoniae caused bronchopneumonia with 24 h. Bacteria were isolated from the lungs in large numbers (greater than 10(5) colony-forming units [CFU] for at least 13 days after inoculation. Thereafter, the viable concentration decreased to about 10(3) CFU at 21 days but increased to 10(4) CFU at 25 days. Mortality rarely exceeded 25%. Plasma zinc concentration decreased, and plasma seromucoid, lysozyme, and alpha2-macrofetoprotein increased during respiratory K. pneumoniae infection in rats. There seemed to be a linear relationship between seromucoid concentration and the concentration of K. pneumoniae in the lung expressed in log10 units. Plasma zinc, alpha2-macrofetoprtoein, or lysozyme levels, however, did not change until the concentration of bacteria retrieved fron lungs exceeded 4 to 5 logs, Analysis of blood samples obtained serially from the orbital sinuses revealed that rats that succumbed to infection had significantly higher levels of seromucoid, alpha2-macrofetoprotein, and lysozyme and lower levels of plasma zinc than infected rats that survived. Progressive increases in seromucoid and particularly in lysozyme and alpha2-macrofetoprotein appeared to be predicative of death. It is postulated that the threshold effect observed for alpha2-macrofetoprotein and lysozyme reflect significant damage to lung tissue, and thus these two variables are good indexes of the severity of this infection. We propose that this model may be of value in elucidating the pathogenesis of respiratory K. pneumoniae as well as in assessing various models of therapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt R. F., Long G. G., Walker J. S. Treatment of respiratory Klebsiella pneumoniae infection in mice with aerosols of kanamycin. Antimicrob Agents Chemother. 1975 Nov;8(5):585–590. doi: 10.1128/aac.8.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Blackburn B. S., Wannemacher R. W., Jr, McGann V. G., Beisel W. R., Dupont H. L. Sequential changes in the concentration of specific serum proteins during typhoid fever infection in man. J Lab Clin Med. 1976 Apr;87(4):577–585. [PubMed] [Google Scholar]
- Canonico P. G., Powanda M. C., Cockerell G. L., Moe J. B. Relationship of serum beta-glucuronidase and lysozyme to pathogenesis of tularemia in immune and nonimmune rats. Infect Immun. 1975 Jul;12(1):42–47. doi: 10.1128/iai.12.1.42-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson E. B., Sanford J. P. The Klebsiella-Enterobacter (Aerobacter)-Serratia group. A clinical and bacteriological evaluation. Medicine (Baltimore) 1967 Jul;46(4):323–340. doi: 10.1097/00005792-196707000-00002. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Marshall L. W., Charache P., Wallace C. K., Melvin V. B. Nosocomial pneumonia. A continuing major problem. Am Rev Respir Dis. 1973 Nov;108(5):1130–1140. doi: 10.1164/arrd.1973.108.5.1130. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E., Friesen A. D., Chou B. Studies on acute phase proteins of rat serum. II. Determination of the contents of 1 -acid glycoprotein, 2-macroglobulin, and albumin in serum from rats suffering from induced inflammation. Can J Biochem. 1972 Aug;50(8):871–880. doi: 10.1139/o72-122. [DOI] [PubMed] [Google Scholar]
- Lindeman R. D., Bottomley R. G., Cornelison R. L., Jr, Jacobs L. A. Influence of acute tissue injury on zinc metabolism in man. J Lab Clin Med. 1972 Mar;79(3):452–460. [PubMed] [Google Scholar]
- Lippman M. E., Finch S. C. A quantitative study of muramidase distribution in normal and nitrogen mustard-treated rats. Yale J Biol Med. 1972 Oct;45(5):463–470. [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Finland M. Infection and antibiotic usage at Boston City Hospital: changes in prevalence during the decade 1964-1973. J Infect Dis. 1974 Apr;129(4):421–428. doi: 10.1093/infdis/129.4.421. [DOI] [PubMed] [Google Scholar]
- Neuhaus O. W., Balegno H. F., Chandler A. M. Induction of plasma protein synthesis in response to trauma. Am J Physiol. 1966 Jul;211(1):151–156. doi: 10.1152/ajplegacy.1966.211.1.151. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R. S., Gee J. B., Finch S. C. Usefulness of serum lysozyme measurement in diagnosis and evaluation of sarcoidosis. N Engl J Med. 1973 Nov 15;289(20):1074–1076. doi: 10.1056/NEJM197311152892007. [DOI] [PubMed] [Google Scholar]
- Pekarek R. S., Burghen G. A., Bartelloni P. J., Calia F. M., Bostian K. A., Beisel W. R. The effect of live attenuated Venezuelan equine encephalomyelitis virus vaccine on serum iron, zinc, and copper concentrations in man. J Lab Clin Med. 1970 Aug;76(2):293–303. [PubMed] [Google Scholar]
- Powanda M. C., Cockerell G. L., Moe J. B., Abeles F. B., Pekarek R. S., Canonico P. G. Induced metabolic sequelae of tularemia in the rat: correlation with tissue damage. Am J Physiol. 1975 Aug;229(2):479–483. doi: 10.1152/ajplegacy.1975.229.2.479. [DOI] [PubMed] [Google Scholar]
- Powanda M. C., Cockerell G. L., Pekarek R. S. Amino acid and zinc movement in relation to protein synthesis early in inflammation. Am J Physiol. 1973 Aug;225(2):399–401. doi: 10.1152/ajplegacy.1973.225.2.399. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., White J. D., Ayala E., Canonico P. G. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect Immun. 1975 Jan;11(1):146–151. doi: 10.1128/iai.11.1.146-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P. Indolent pulmonary abscess associated with Klebsiella and Enterobacter. Am Rev Respir Dis. 1973 Jun;107(6):1055–1059. doi: 10.1164/arrd.1973.107.6.1055. [DOI] [PubMed] [Google Scholar]
- Rose H. D., Heckman M. G., Unger J. D. Pseudomonas aeruginosa pneumonia in adults. Am Rev Respir Dis. 1973 Mar;107(3):416–422. doi: 10.1164/arrd.1973.107.3.416. [DOI] [PubMed] [Google Scholar]
- Sarcione E. J. Synthesis and secretion of alpha 2-(acute phase) globulin by the isolated perfused liver from injured adult rats. Biochemistry. 1970 Jul 21;9(15):3059–3062. doi: 10.1021/bi00817a019. [DOI] [PubMed] [Google Scholar]
- Sharbaugh R. J., Rambo W. M. Serum prognostic indicators in experimental Bacteroides peritonitis. Arch Surg. 1975 Sep;110(9):1146–1149. doi: 10.1001/archsurg.1975.01360150090016. [DOI] [PubMed] [Google Scholar]
- Tillotson J. R., Finland M. Bacterial colonization and clinical superinfection of the respiratory tract complicating antibiotic treatment of pneumonia. J Infect Dis. 1969 Jun;119(6):597–624. doi: 10.1093/infdis/119.6.597. [DOI] [PubMed] [Google Scholar]
- Tillotson J. R., Lerner A. M. Pneumonias caused by gram negative bacilli. Medicine (Baltimore) 1966 Jan;45(1):65–76. doi: 10.1097/00005792-196601000-00003. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, DuPont H. L., Pekarek R. S., Powanda M. C., Schwartz A., Hornick R. B., Beisel W. R. An endogenous mediator of depression of amino acids and trace metals in serum during typhoid fever. J Infect Dis. 1972 Jul;126(1):77–86. doi: 10.1093/infdis/126.1.77. [DOI] [PubMed] [Google Scholar]
- Weimer H. E., Benjamin D. C. Immunochemical detection of an acute-phase protein in rat serum. Am J Physiol. 1965 Oct;209(4):736–744. doi: 10.1152/ajplegacy.1965.209.4.736. [DOI] [PubMed] [Google Scholar]
- Werner M. Serum protein changes during the acute phase reaction. Clin Chim Acta. 1969 Aug;25(2):299–305. doi: 10.1016/0009-8981(69)90272-1. [DOI] [PubMed] [Google Scholar]
- Woodward J. M., Camblin M. L., Jobe M. H. Influence of bacterial infection on serum enzymes of white rats. Appl Microbiol. 1969 Jan;17(1):145–149. doi: 10.1128/am.17.1.145-149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh R. A., Barrett B., Niemirowski L., Fiorella B. J. Turnover rate of orosomucoid in the dog with sterile abscess. Am J Physiol. 1972 May;222(5):1326–1332. doi: 10.1152/ajplegacy.1972.222.5.1326. [DOI] [PubMed] [Google Scholar]