Abstract
Rationale: Effective therapeutic interventions for chronic, idiopathic lung diseases remain elusive. Normalized T-cell function is an important contributor to spontaneous resolution of pulmonary sarcoidosis. Up-regulation of inhibitor receptors, such as programmed death-1 (PD-1) and its ligand, PD-L1, are important inhibitors of T-cell function.
Objectives: To determine the effects of PD-1 pathway blockade on sarcoidosis CD4+ T-cell proliferative capacity.
Methods: Gene expression profiles of sarcoidosis and healthy control peripheral blood mononuclear cells were analyzed at baseline and follow-up. Flow cytometry was used to measure ex vivo expression of PD-1 and PD-L1 on systemic and bronchoalveolar lavage–derived cells of subjects with sarcoidosis and control subjects, as well as the effects of PD-1 pathway blockade on cellular proliferation after T-cell receptor stimulation. Immunohistochemistry analysis for PD-1/PD-L1 expression was conducted on sarcoidosis, malignant, and healthy control lung specimens.
Measurements and Main Results: Microarray analysis demonstrates longitudinal increase in PDCD1 gene expression in sarcoidosis peripheral blood mononuclear cells. Immunohistochemistry analysis revealed increased PD-L1 expression within sarcoidosis granulomas and lung malignancy, but this was absent in healthy lungs. Increased numbers of sarcoidosis PD-1+ CD4+ T cells are present systemically, compared with healthy control subjects (P < 0.0001). Lymphocytes with reduced proliferative capacity exhibited increased proliferation with PD-1 pathway blockade. Longitudinal analysis of subjects with sarcoidosis revealed reduced PD-1+ CD4+ T cells with spontaneous clinical resolution but not with disease progression.
Conclusions: Analogous to the effects in other chronic lung diseases, these findings demonstrate that the PD-1 pathway is an important contributor to sarcoidosis CD4+ T-cell proliferative capacity and clinical outcome. Blockade of the PD-1 pathway may be a viable therapeutic target to optimize clinical outcomes.
Keywords: programmed death-1, programmed death-L1, sarcoidosis, proliferation, gene expression
At a Glance Commentary
Scientific Knowledge on the Subject
Enhanced inhibitory receptor expression has been reported in lung cancers, but its role in idiopathic lung disease, such as sarcoidosis, is unknown. Recent studies demonstrate global CD4+ T-cell dysfunction among subjects with chronic sarcoidosis. Little is known regarding the contribution of inhibitory receptors, such as programmed death-1 (PD-1), to sarcoidosis CD4+ T-cell proliferative capacity.
What This Study Adds to the Field
This investigation demonstrates that increased PD-1+ CD4+ T cells are present in patients with sarcoidosis, both at systemic levels and within local environments, leading to decreases in cellular proliferative capacity. Blockade of the PD-1 pathways restores CD4+ T-cell proliferative capacity to levels of healthy control subjects. Longitudinal analysis demonstrates increased PD+ CD4+ T cells during active disease but normalized PD-1 expression and proliferative capacity in subjects with sarcoidosis with spontaneous resolution. These findings indicate that the inhibitory receptor, PD-1, carries immunologic and clinical significance in sarcoidosis pathogenesis.
Despite the increasing incidence in idiopathic, chronic inflammatory lung diseases, such as sarcoidosis (1, 2), identification of effective therapeutics remains elusive. Clinical trials and genetic and immunologic studies support the concept that immunoregulation makes important contributions to outcome in idiopathic pulmonary diseases. Genomic-wide association studies for molecular determinants of sarcoidosis severity have identified candidate genes associated with immune recognition and regulation (3–7), T-cell receptor signaling, and JAK/STAT pathway, including members of the CD28 family (8, 9). In a cohort of 89 patients with idiopathic pulmonary fibrosis, down-regulation of CD28 in circulating CD4+ T cells has been shown to be a marker of poor prognoses, findings recently confirmed at the peripheral blood mononuclear cell (PBMC) gene expression level in two independent idiopathic pulmonary fibrosis cohorts (10, 11). In pulmonary sarcoidosis, reversal of global CD4+ subset dysfunction has been associated with spontaneous clinical resolution (12).
Regulation of T-cell function is orchestrated by costimulatory and coinhibitory pathways. Inhibitory receptors, such as programmed death-1 (PD-1), negatively impact CD4+ T-cell function (13, 14). When PD-1 is engaged, Src homology phosphatase-1 and -2 block phosphatidylinositol 3 kinase/Akt (PI3K/Akt) activation and induce both CD3- and CD28-mediated alterations to the T-cell transcriptome leading to reduced T-cell activation (15, 16). Blockade of the PD-1 pathway restores T-cell function in granulomatous diseases, such as chronic beryllium disease (CBD) (17). Blockade of the PD-1 pathway also leads to clinical improvement of lung cancers (18, 19). We report for the first time that the presence of immunosuppressive microenvironments characterized by PD-1 ligand (PD-L1) up-regulation within sarcoidosis granulomas. Investigation of sarcoidosis T cells reveals increased PD-1+ CD4+ T cells with reduced proliferative capacity; blockade of the PD-1 pathway normalized their proliferative capacity. Analysis of subjects with sarcoidosis with spontaneous clinical resolution reveals reduced PD-1+ CD4+ T cells and normal T-cell proliferative capacity, whereas subjects experiencing clinical progression possess increased PD-1+ CD4+ T cells with reduced proliferative capacity, suggesting the PD-1 up-regulation carries immunologic and clinical significance to sarcoidosis pathogenesis.
Methods
Study Population
For inclusion in this study, the clinical and radiographic criteria used to define sarcoidosis were applied (20, 21). Patients from the Cleveland Clinic and Vanderbilt University Medical Center were enrolled; ∼40% of subjects had participated in a previous investigation (12). All subjects provided written informed consent that was approved by the appropriate Institutional Review Boards. There were four subject cohorts: disease control, healthy control, subjects with sarcoidosis with active disease, and subjects with sarcoidosis with resolved disease. The disease control subjects were symptomatic subjects with diagnoses such as malignancy, interstitial lung disease, or fungal infections. The subjects with sarcoidosis had either active disease, (characterized by reductions in FVC, radiographic progression, or acceleration of pulmonary symptoms) or resolved disease (characterized by normalized FVC or chest radiograph or resolution of pulmonary symptoms despite being off immunosuppressive therapy). Each disease control subject had evidence of active disease. Study subject demographics are provided in Table 1 for peripheral blood and Table 2 for bronchoalveolar lavage (BAL). The clinical diagnosis, disease activity, and presence of immunosuppressive therapy for the BAL subjects is provided in Table E1 in the online supplement.
Table 1.
Demographics of Peripheral Blood Sarcoidosis and Control Populations
| Sarcoidosis | Healthy Control Subjects | |
|---|---|---|
| Number | 77 | 40 |
| Sex, female; male | 50; 27 | 33; 7 |
| Age, median (min, max), yr | 49 (22, 73) | 36 (23, 64) |
| Race | 32 AA; 45 W | 1 A; 14 AA; 23 W; 1 H;1 AI |
| PBMC CD4/CD8 ratio | 3.59 | 2.65 |
Definition of abbreviations: A = African; AA = African American; AI = American Indian; H = Hispanic; PBMC = peripheral blood mononuclear cells; W = white.
Table 2.
Demographics of Bronchoalveolar Lavage Sarcoidosis and Disease Control Populations
| Sarcoidosis | Disease Control Subjects | Healthy Control Subjects | |
|---|---|---|---|
| Number | 20 | 9 | 4 |
| Sex, female; male | 11; 9 | 4; 5 | 3; 1 |
| Age, median (min, max), yr | 44 (25, 67) | 51 (38, 62) | 41 (38, 54) |
| Race | 1A; 6 AA; 13 W | 1 AA; 8 W | 4 W |
| BAL CD4/CD8 ratio | 7.79 | 4.43 | 3.38 |
Definition of abbreviations: A = African; AA = African American; BAL = bronchoalveolar lavage; W = white.
Cell Isolation
BAL fluid and peripheral blood were processed as previously described (22, 23). CD4+ T cells were isolated from PBMC using Dynabeads CD4+ isolation kit according to the manufacturer’s protocol (Invitrogen, Grand Island, NY).
Microarray Analysis
The microarray gene expression dataset, GSE1907, was downloaded from the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) (24). Data were analyzed using Significant Analysis of Microarrays, as previously described (25). A stringent false-discovery rate cutoff of less than 1% was used to define statistical significance, using PCluster to depict microarrays analysis results (26).
Immunohistochemistry
Diagnostic tissue blocks obtained from patients with sarcoidosis were provided by the Vanderbilt Translational Pathology Shared Resource with approval from the Vanderbilt Institutional Review Board. Negative control lung specimens were obtained from subjects who expired from nonpulmonary disease with no lung pathology; lung cancer biopsies served as positive controls for PD-1 and PD-L1 expression. Immunohistochemistry for PD-1 and PD-L1 were conducted as previously described (27), using anti–PD-L1 (clone 29E.2A3, BioLegend, San Diego, CA), and anti–PD-1 (clone 7A11B1, Sigma-Aldrich, St. Louis, MO). The Bond Refine Polymer detection system was used for visualization, after hematoxylin and eosin staining.
Flow Cytometry
Anti-CD3, anti-CD4, anti-CD45RO, anti-CCR7, T regulatory cells, anti–PD-1, anti–PD-L1, anti–PD-L2, anti-CD11c, anti-CD19, and anti-CD14 were obtained from BD Biosciences (San Jose, CA). Surface staining of cells was performed as previously described (12). Th17 cells were identified using flow cytometry as previously described (28). Cells were gated on singlets, live CD3+, and CD4+ cells. All flow cytometry experiments were acquired with a LSR-II flow cytometer (BD Biosciences). Live cells were gated based on forward and side scatter properties, and analysis was performed using FlowJo software (Tree Star, Ashland, OR). A minimum of 30,000 events were acquired per sample.
Proliferation Assay and In Vitro Blockade of PD-1 Pathway
For the blockade experiments, PBMC were labeled with carboxyfluorescein succinimidyl ester as previously described (23), then incubated overnight with or without the combination of anti–PD-1(5 μg/ml, J116; eBioscience, San Diego, CA), anti–PD-L1(2 μg/ml, MIH1; eBioscience), and anti–PD-L2 (2 μg/ml, MIH18; eBioscience) blocking antibodies in RPMI 1640-supplemented medium before stimulation with anti-CD3 and anti-CD28 antibodies. Cells were then stimulated with plate-bound anti-CD3 antibody (OKT-3; American Type Culture Collection, Manassas, VA) and soluble anti-CD28 antibody (1 μg/ml, BD Biosciences) at a concentration of 2 × 106/ml for 5 days.
Statistical Analysis
Pearson correlation and Student t distribution were used to identify statistical significance in microarray analysis. Comparisons between immunologic cohorts were performed using an unpaired two-tailed Student t test. Multiple-group comparisons were performed using a one-way analysis of variance. Proliferation data were analyzed using the Mann-Whitney U test. All statistical analyses were performed using Prism version 6.0 (GraphPad software). A P value of less than 0.05 was considered statistically significant.
Results
Microarray Analysis Demonstrates Overexpression of PDCD1 in Sarcoidosis PBMC
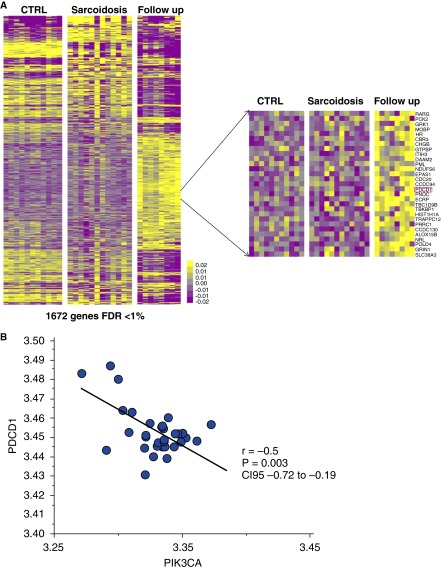
A microarray gene expression dataset was downloaded from the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) under the series accession number GSE1907. In this study, total RNA was extracted from PBMC and hybridized to Affymetrix GeneChip microarrays in 12 healthy control subjects and 12 subjects with sarcoidosis at baseline (7 subjects with stage I and 5 subjects with stage II/III disease) and in 8 of these 12 subjects after 6 months’ follow-up (5 subjects with stage I and 3 subjects with stage II/III disease) (24). We identified 1,672 differentially expressed genes (false-discovery rate < 1%) among healthy control subjects, subjects with sarcoidosis at baseline, and subjects with sarcoidosis after follow-up (Figure 1A). PDCD1, the gene encoding for PD-1, was part of this gene signature and among the overexpressed genes in subjects with sarcoidosis after follow-up (Figure 1A). Interestingly, we also identified that PIK3CA, the gene that encodes a class 2 PIK3 and located downstream of PD-1, was also among this signature and significantly underexpressed in subjects with sarcoidosis after follow-up; PIK3CA was also negatively correlated with PDCD1 (r = −0.5; P = 0.003; 95% confidence interval, −0.72 to −0.19) (Figure 1B), confirming the downstream effects of PD-1 activation at the systemic gene expression level in sarcoidosis.
Figure 1.
Semisupervised clustering heat map demonstrates differentially expressed gene expression patterns in control subjects and subjects with sarcoidosis at baseline and after follow-up. (A) Semisupervised clustering demonstrates the patterns of expression of 1,672 differentially expressed genes among healthy control subjects (n = 12) and subjects with sarcoidosis at baseline (n = 12) and after follow-up (n = 8). Every column represents a sample from a subject and every row, a gene. Color scale is shown next to heat map in log2 scale; yellow denotes increased expression over the geometric mean of samples, and purple, decrease. (Inset) PDCD1, the gene encoding for PD-1, is among the overexpressed genes in subjects with sarcoidosis after follow-up. (B) Correlation between log2 transformed microarray expression levels of PDCD1 (y axis) and PIK3CA (x axis) among all the microarray samples in the study.
Patients with Sarcoidosis Have Increased PD-1 Expression on Peripheral CD4+ T Cells
We first examined PD-1 expression by peripheral CD4+ T cells from patients with sarcoidosis. PBMC were obtained from healthy control subjects (n = 40) and patients with sarcoidosis (n = 77) (Table 1). Flow cytometry analysis of unstimulated CD4+ T cells from PBMC shows that patients with sarcoidosis have a significantly higher percentage of PD-1–expressing CD4+ T cells than healthy control subjects (P < 0.0001, two-tailed t test) (Figure 2A). The CD4+ T cells also demonstrated distinctions in spontaneous IL-2 and IFN-γ expression between sarcoidosis and healthy control subjects, as previously described (29, 30) (see Figures E1 and E2 in the online supplement). Because up-regulated PD-1 expression naturally occurs with T-cell demise, we determined whether the expression of PD-1 is associated with the expression of other memory T-cell markers. Using CCR7 and CD45RO to identify CD4+ memory T-cell subsets, we evaluated PD-1 expression on naive, effector memory (TEM), terminal effector memory (TEMRA), and central memory (TCM) cells in the blood. Distribution of CD4+ memory T-cell subsets did not differ between control subjects and patients with sarcoidosis; however, there were distinctions in the naive population subset (P < 0.0001) (Figure 2B). Healthy control subjects possessed a significantly higher quantity of naive cells than subjects with sarcoidosis. The percentages of TCM and TEM cells expressing PD-1 were significantly greater in subjects with sarcoidosis (P = 0.02 and 0.03, respectively) (Figure 2C). We then analyzed Th17 cells, a distinct population of effector cells, from subjects with sarcoidosis for PD-1 expression. PD-1 expression was also increased on sarcoidosis Th17 cells (P = 0.001) (Figure 2D).
Figure 2.
Increased percentage of programmed death-1 (PD-1)+ CD4+ T cells from the blood of patients with sarcoidosis. (A) Percentages of total peripheral CD4+ T cells expressing PD-1 from healthy control subjects (HC, n = 40) and patients with sarcoidosis (S, n = 77). PD-1 was determined by gating on the naive population (shaded) for each sample as shown in the representative histograms. Overlay represents one HC (gray line) and one subject with sarcoidosis (black line). (B) Distribution of CD4+ memory T-cell subsets and (C) the percentage of CD4+ memory T-cell subsets expressing PD-1 from healthy control subjects (n = 23) and patients with sarcoidosis (n = 47). Memory subsets were determined as shown in the representative dot plots. (D) Percent PD-1+ unstimulated CD4+ T cells from peripheral blood mononuclear cells (PBMC) of healthy control subjects (n = 7) and patients with sarcoidosis (n = 17). Additionally for the patients with sarcoidosis, PD-1 was measured on Tregulatory (n = 5) and CD4+IL-17A+ T cells from polyclonally activated PBMC (n = 17). Measurement was not performed on healthy control CD4+IL-17A+ cells due to low number of cells. *P < 0.0001; **P < 0.05. CM = central memory T cells; EM = effector memory T cells; N = naive T cells; TEM = terminal effector memory T cells.
PD-1 expression in sites of active sarcoidosis involvement.
Patients with sarcoidosis are known to have relative anergy when systemic cells are assessed versus local immune cells. To determine if the effects of PD-1 on PBMCs reflected local disease activity, we assessed the expression of PD-1 on BAL-derived CD4+ T cells. Compared with the periphery, there is a significant increase in PD-1 expression on BAL CD4+ T cells (P < 0.0001) (Figure 3A). Similar distinctions were noted with comparison of systemic and BAL-derived Th17 and T regulatory cells (Figures E3 and E4). Comparison of expression in local and systemic CD4+ T cells derived from the same subjects confirmed that the percentage of PD-1+ CD4+ T cells was greater locally (P < 0.0001) (Figure 3B). Comparison of percentage of PD-1+ CD4+ T cells in sarcoidosis BAL compared with BAL of disease control subjects revealed greater PD-1 expression in the control cohort than the sarcoidosis cohort (P = 0.004) (Figure 3C). There was no significant difference in percentage of PD-1+ CD4+ T cells in the BAL of disease control subjects and subjects with sarcoidosis with active disease; however, subjects with sarcoidosis with disease resolution have significantly lower PD-1 expression when compared with disease control subjects or with subjects with sarcoidosis with active disease (P < 0.0001 and P = 0.04) (Figure 3D). No significant difference was found between healthy control subjects and subjects with sarcoidosis with disease resolution (Figure 3D). Similarly, no distinctions were detected between disease control subjects and subjects with sarcoidosis with active disease. Subjects with sarcoidosis with active disease have distinct percentages of PD-1+ CD4+ T cells from healthy control subjects (P = 0.04). Analysis of sarcoidosis CD4+ T-cell subsets in the BAL revealed a significant difference between healthy control subjects and Th17 cells as well (P < 0.001) but not T regulatory cells (Figure 3E). Systemically, distribution of CD4+ memory T-cell subsets or the percentage of cells expressing PD-1 did not differ between disease control subjects and patients with sarcoidosis (Figures E5 and E6).
Figure 3.
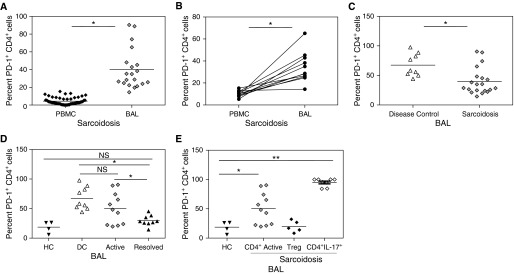
Programmed death-1 (PD-1) expression in bronchoalveolar lavage (BAL) from patients with sarcoidosis. (A) Comparison of PD-1 expression on CD4+ T cells in the periphery (peripheral blood mononuclear cells [PBMC], n = 65) and BAL (n = 20) of patients with sarcoidosis. (B) Percent PD-1+ CD4+ cells in peripheral and local environments of 10 patients with sarcoidosis. (C) BAL from disease control subjects (DC, n = 9) and patients with sarcoidosis (S, n = 20). (D) Percent PD-1+ CD4+ memory T-cell subsets in BAL according to sarcoidosis clinical disease activity. BAL, 11 subjects during active disease, 9 subjects with resolved disease, 9 disease control subjects (DC), and 4 healthy control subjects (HC). (E) Percent PD-1+ CD4+, Th17, and T regulatory cells. *P < 0.001; **P < 0.05.
Expression of PD-L1 and PD-L2 on sarcoidosis cell subsets.
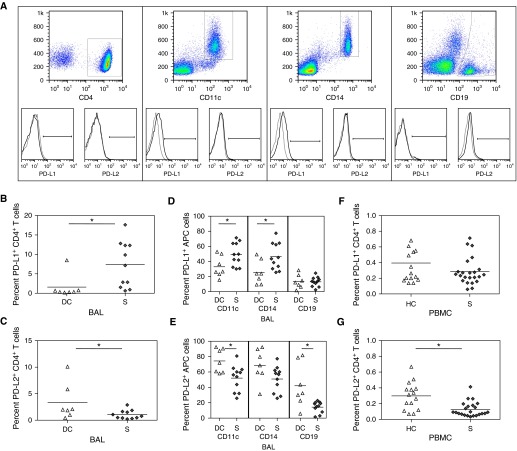
Suppression of T-cell function by the PD-1 requires engagement by its ligands, PD-L1 or PD-L2, on antigen-presenting cells (APC). Using flow cytometry, we compared expression of PD-1 ligands, PD-L1 and PD-L2, on CD4+ T cells, as well as on sarcoidosis APCs, specifically monocytes, B cells, and CD11c+ cells that could include dendritic cells and macrophages. Investigation of the local percentage of PD-L1+ CD4+ T cells in disease control BAL versus sarcoidosis BAL revealed a significantly higher percentage in the sarcoidosis BAL (P = 0.03). The disease control BAL demonstrated significantly higher PD-L2+ CD4+ T cells than subjects with sarcoidosis (P = 0.05) (Figures 4A–4C).
Figure 4.
Programmed death-1 ligand (PD-L1) expression on peripheral cells. (A) Gating strategy for the CD4+, CD11c+, CD14+, and CD19+ cells and representative PD-L1 histograms for each set. (B) Percent PD-L1+ and (C) percent PD-L2+ CD4+ T cells from bronchoalveolar lavage (BAL) of disease control subjects (DC, n = 7) and patients with sarcoidosis (S, n = 11). (D) Percent PD-L1+ and (E) percent PD-L2+ antigen-presenting cells from BAL of disease control subjects (DC, n = 7) and patients with sarcoidosis (S, n = 11). (F) Percent PD-L1+ and (G) percent PD-L2+ CD4+ T cells from BAL of healthy control subjects (HC, n = 15) and patients with sarcoidosis (S, n = 23) *P ≤ 0.05.
Investigation of the local percentage of PD-L1+ CD11c+ and CD14+ cells in disease control BAL versus sarcoidosis BAL revealed a significantly higher percentage in the sarcoidosis BAL (P = 0.04 and 0.02, respectively). There was no distinction in the B-cell population (Figure 4D). The percentage of APCs expressing PD-L2 was significantly higher in the disease control CD11c+ and CD19+ cell population (P = 0.01 and 0.006, respectively), while approaching significance in the macrophage population (P = 0.07) (Figure 4E). Systemic assessment for these same inhibitory receptor ligands revealed no distinctions in PD-L1 expression on sarcoidosis CD4+ T cells when compared with healthy control subjects (Figure 4F); however, there was a significantly higher percentage of systemic PD-L2+ CD4+ T cells among healthy control subjects than subjects with sarcoidosis (P = 0.003) (Figure 4G). In addition, there were no differences between sarcoidosis and healthy control subjects in PD-L1 or PD-L2 expression among the APC populations (Figures 4H and 4I).
Sarcoidosis granulomas demonstrate PD-L1 up-regulation.
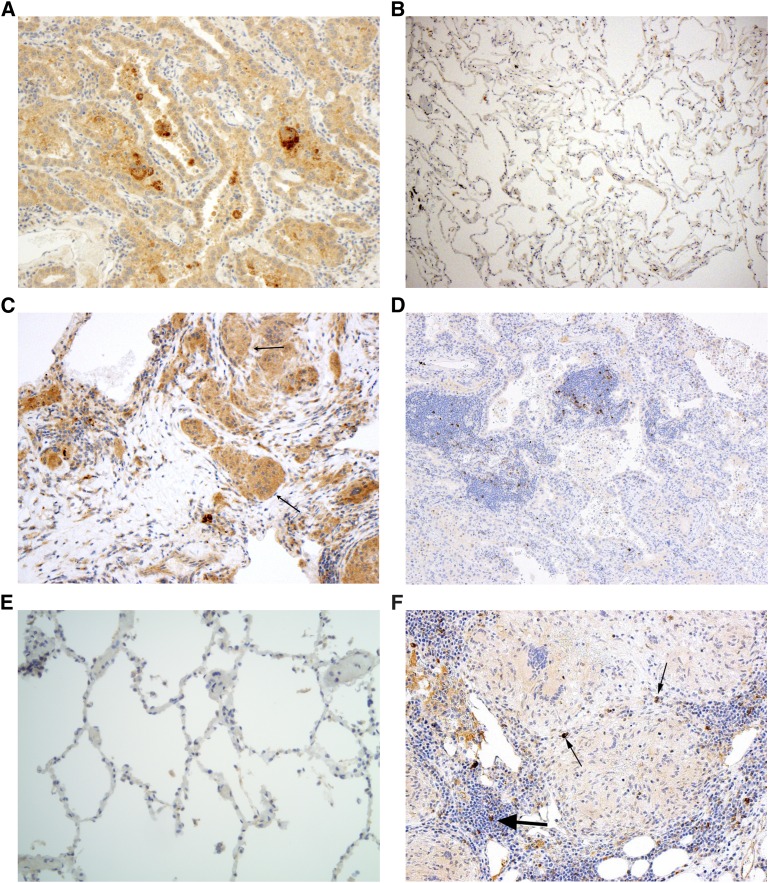
Sarcoidosis involvement of the lung is characterized by extensive CD4+ and CD8+ T-cell alveolitis, macrophage infiltration, and granuloma formation. To determine whether the PD-1and PD-L1 up-regulation contributes to sarcoidosis granuloma formation, we conducted immunohistochemistry analysis for PD-1 and PD-L1 on sarcoidosis lung specimens. Lung biopsies of pulmonary malignancy served as the positive control and lung biopsies from healthy subjects as the negative control. Immunohistochemistry analysis for PD-L1 expression revealed no PD-L1 up-regulation in healthy control lung; there was increased PD-L1 expression within pulmonary malignancies along the epithelial border as well as within sarcoidosis granulomas (Figures 5A–5C).
Figure 5.
Immunohistochemistry analysis for programmed death-1 (PD-1) and its ligand, PD-L1, expression in pulmonary specimens. (A–C) Assessment for PD-L1 expression in pulmonary malignancy (n = 7), healthy lung specimens (n = 3), and sarcoidosis (n = 22). (D–F) Assessment for PD-1 expression in pulmonary malignancy (n = 7), healthy lung specimens (n = 3), and sarcoidosis (n = 22). A representative section of lung cancer, healthy lung, and pulmonary sarcoidosis for PD-L1 and PD-1 expression is shown. Scant PD-1 expression is noted in sarcoidosis granulomas (thin arrows), with higher PD-1 expression in bronchial associated lymphoid tissue (thick arrows).
PD-1 expression was present on some lymphocytes surrounding areas of malignant transformation in lung cancer specimens and none on lymphocytes within healthy lung (Figures 5D and 5E). Similarly, there was scant PD-1 expression on lymphocytes within the sarcoidosis granulomas (see arrows); however, notable expression was present on lymphocytes within the bronchial associated lymphoid tissue (Figures 5F). Thus, in assessing for expression of members of the PD-1 pathway, both sarcoidosis and lung cancer specimens demonstrated high PD-L1 expression—within multinuclear giant cells of sarcoidosis specimens and sites of malignant transformation of lung cancer specimens. Both sarcoidosis and lung cancer specimens demonstrate scant PD-1 expression, but in lymphoid areas surrounding either sarcoidosis granulomas or malignant transformation, PD-1 expression is high (Figure 5).
PD-1 pathway blockade enhances sarcoidosis CD4+ proliferative capacity.
Cellular proliferation makes important contributions to effective adaptive immune responses. PD-1 up-regulation has been implicated in loss of CD4+ T-cell function, specifically diminished proliferative capacity and cytokine expression. We assessed if PD-1 up-regulation carried functional significance. Diminished proliferative capacity was noted in sarcoidosis CD4+ T cells compared with those observed in healthy control subjects (P < 0.01) (Figure 6A). Using antibodies against PD-1, PD-L1, and PD-L2, we investigated if blockade of the PD-1 pathway enhanced sarcoidosis CD4+ T-cell proliferative capacity. Blockade of the pathway in CD4+ T cells from healthy control subjects did not result in enhanced proliferative capacity, (P = 0.79) (Figure 6B). There were subjects with sarcoidosis with proliferative capacity greater than 50%, which is akin to the capacity observed in healthy control subjects; blockade of the PD-1 pathway in sarcoidosis CD4+ T cells with greater than 50% proliferative capacity did not result in enhanced proliferative capacity (P = 0.86) (Figure 6C). However, blockade of the PD-1 pathway in sarcoidosis CD4+ T cells with proliferative capacity of less than 50% of healthy control subjects resulted in a considerable increase in proliferative capacity (P = 0.0003) (Figure 6D). In fact, blockade of the PD-1 pathway resulted in such a substantial increase in sarcoidosis T-cell proliferative capacity that distinctions between sarcoidosis and healthy control proliferative capacity were no longer evident (P = 0.09) (Figure 6E).
Figure 6.
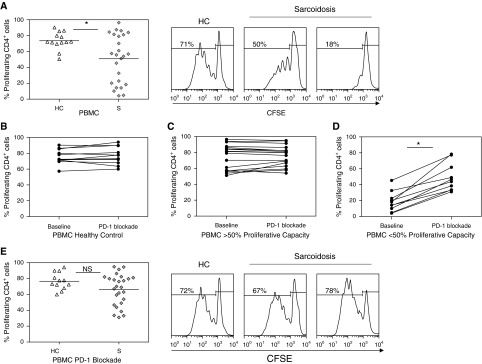
Blockade of programmed death-1 (PD-1) pathway increases sarcoidosis CD4+ T-cell proliferative capacity in vitro. (A) Percent CD4+ T-cell proliferation of healthy control subjects (HC, n = 13) and patients with sarcoidosis (S, n = 25) after in vitro T-cell receptor stimulation. Representative proliferation data for one HC, one subject with sarcoidosis with high proliferative capacity (>50%), and one subject with sarcoidosis with low proliferative capacity (<50%) at baseline. (B) Percent proliferation of healthy control (n = 12) CD4+ T cells at baseline after stimulation and with the addition of anti–PD-1, anti–PD-L1, and anti–PD-L2 blocking antibodies (PD-1 blockade). Percent proliferation of sarcoidosis CD4+ T cells with (C) greater than 50% baseline proliferation after stimulation (n = 17), and (D) less than 50% baseline proliferation (n = 10) after the addition of anti–PD-1, anti–PD-L1, and anti–PD-L2 blocking antibodies (PD-1 blockade). (E) Combined percent proliferation of healthy control subjects (HC, n = 12) and patients with sarcoidosis (S, n = 26) after blocking the PD-1 pathway. Representative proliferation data after PD-1 blockade for one HC, one sarcoidosis subject with high baseline proliferative capacity (>50%), and one sarcoidosis subject with low baseline proliferative capacity (<50%). *P < 0.01. CFSE = carboxyfluorescein succinimidyl ester; PBMC = peripheral blood mononuclear cells.
Down-regulation of PD-1 expression on CD4+ T cells accompanies spontaneous clinical resolution of pulmonary sarcoidosis.
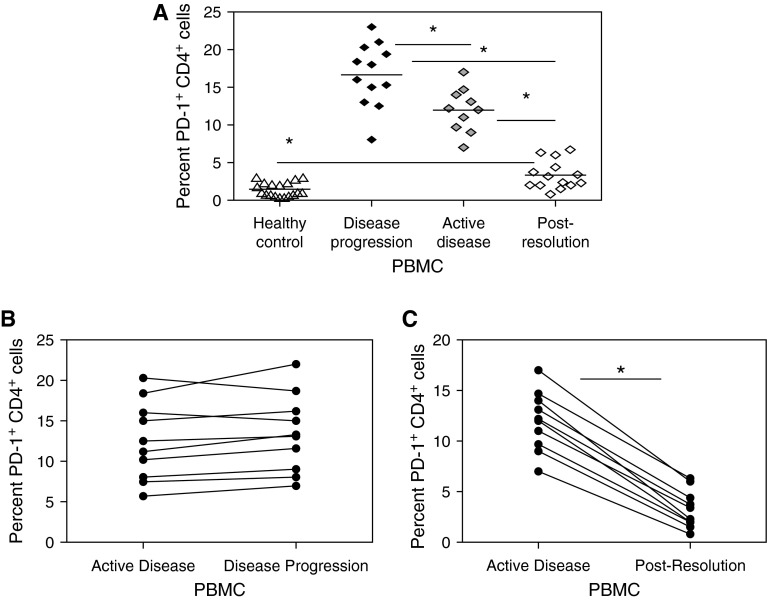
Subjects with sarcoidosis with spontaneous clinical resolution exhibit restoration of CD4+ T-cell proliferative capacity, whereas persistent loss of cellular function was noted in subjects experiencing disease progression (12). Building on the observation that PD-1 pathway blockade restores sarcoidosis CD4+ T-cell proliferative capacity (Figure 6), we conducted a longitudinal assessment of baseline PD-1 expression in subjects with sarcoidosis at the time of diagnosis or disease activity, during disease progression, or at spontaneous resolution, comparing each clinical stage to healthy control subjects.
During active disease, CD4+ T cells expressing PD-1 were five to six times higher in subjects with sarcoidosis than healthy control subjects (P < 0.0001) (Figure 7A). There was a significant decline in PD-1 expression on the CD4+ T cells of subjects experiencing spontaneous clinical resolution, when compared with expression among CD4+ T cells of subjects with active disease (P < 0.0001) (Figure 7A). The decline in PD-1 expression in subjects who spontaneously resolved their disease resulted in PD-1 levels that remained distinct from healthy control subjects (P = 0.0008) as well as those with disease progression (P < 0.0001) (Figure 7A). Their CD4+ T-cell proliferative capacity was restored within range of healthy control subjects (data not shown). Subjects experiencing disease progression demonstrated persistent elevation of PD-1 expression on their CD4+ T cells (Figure 7B). Investigation of CD4+ T cells during active disease and after disease resolution demonstrated a significant increase in PD-1+ CD4+ T cells during active disease; however, a significant decline was present with disease resolution (P < 0.0001) (Figure 7C).
Figure 7.
Percent programmed death-1 (PD-1)+ CD4+ T cells decreases when sarcoidosis disease resolves. (A) Percent PD-1+ CD4+ T cells from healthy control subjects (18), subjects with sarcoidosis with disease progression (12), active disease (10) and clinical resolution (14). (B) Ten representative subjects with sarcoidosis disease progression, and (C) 10 subjects during active disease and after disease resolution. *P < 0.001. PBMC = peripheral blood mononuclear cells.
Discussion
This is the first investigation to demonstrate increased numbers of PD-1+ CD4+ T cells in subjects with sarcoidosis. These cells were present systemically, as well as locally in sarcoidosis BAL-derived fluid. Sarcoidosis granulomas demonstrated PD-L1 up-regulation, which was absent from healthy lung tissue. Furthermore, blockade of the PD-1 pathway restored the proliferative capacity of sarcoidosis CD4+ T cells to levels consistent with healthy control subjects, suggesting that PD-1 up-regulation is an important contributor to loss of sarcoidosis proliferative capacity. These observations demonstrate that PD-1 expression carries immunologic consequences in sarcoidosis pathogenesis.
We noted significantly higher percentages of PD-1+ CD4+ T cells in sarcoidosis BAL, compared with systemic T cells (Figure 3). We also noted in these same BAL CD4+ T cells spontaneous expression of IL-2 and IFN-γ (Figures E1 and E2). We previously reported that, despite spontaneous expression of Th1 cytokines, sarcoidosis BAL-derived CD4+ T cells demonstrate reduced proliferative capacity and Th-1 cytokine expression after T-cell receptor stimulation (12). This observation of spontaneous cytokine expression, reduced T-cell function, and elevated inhibitory receptor expression has been well described in several inflammatory conditions driven by microbial antigens (31–33). The lung is a unique environment with constant exposure to foreign antigens. Mycobacterial antigens or signals consistent with mycobacterial antigens have been localized to sarcoidosis granulomas (34, 35) The distinction in local versus peripheral PD-1 expression could reflects distinction in microbial antigen load, which has been shown to affect immune function and drive PD-1 up-regulation (36). Thus, the observation of high spontaneous cytokine production and increased PD-1 expression likely reflects the consequences of the host immune system attempting to clear persistent, foreign antigen while minimizing organ destruction. PD-1 up-regulation has been shown to serve as a protective effect against organ destruction during conditions of high antigenic burden. It has been shown that higher frequencies of T cells, monocytes, and B cells expressing PD-1 and its ligand(s) are present among patients with tuberculosis. In vitro PD-1 blocking rescued Mycobacterium tuberculosis–specific IFN-γ–producing T cells from undergoing apoptosis. The number of PD-1–expressing T cells decreased significantly during therapy and inversely correlated with IFN-γ–dominant T-cell response against M. tuberculosis, thus demonstrating the protective effects of the PD-1 pathway during tuberculosis infection (37). Also in tuberculosis murine models, reduction of bacterial burden through administration of chemotherapy led to significant reductions in PD-1 expression on T cells (38).
This is also the first report to suggest a positive correlation between the local and systemic numbers of PD-1+ CD4+ T cells and sarcoidosis disease activity (Figures 3 and 7). It is interesting that control subjects and subjects with sarcoidosis had similar expression of the PDCD1 gene at baseline; however, increased PDCD1 expression occurred in the subjects with sarcoidosis at follow-up (Figure 1). Comparison of local PD-1+ CD4+ T-cell expression revealed a significantly higher expression in disease control subjects compared with subjects with sarcoidosis. This distinction disappeared when subjects with sarcoidosis were separated based on the clinical phenotypes of active disease versus resolution (Figure 3). The lack of distinction between disease control subjects and subjects with sarcoidosis with active disease most likely reflects high percentages of antigen burden, either due to microorganisms such as histoplasmosis, to mycobacterial antigens, or to high tumor burden. Antigen load has been shown to regulate PD-1 expression (36). It is possible that as subjects with sarcoidosis resolve their granulomas and remove the mycobacterial antigens shown to be within them, PD-1 expression normalizes. Longitudinal analysis of sarcoidosis PBMC demonstrates increased numbers of PD-1+ CD4+ T cells in all subjects at the time of diagnosis and during active disease. Those experiencing disease progression continue to have high numbers systemically, whereas those with disease resolution have PD-1+ CD4+ T-cell levels analogous to those of healthy control subjects (Figure 7). This correlation of the presence of PD-1+ T cells with disease progression has been previously reported in patients with cancer and HIV (39, 40). PD-1 up-regulation has been described in other granulomatous diseases, such as on beryllium-specific CD4+ T cells in CBD. In the subjects with CBD, PD-1 expression correlated with the severity of the T-cell alveolitis (17). PD-1 has also been investigated specifically in the context of immune regulation, using PD-1−/− gene disrupted M. tuberculosis–infected mice (41). This model demonstrated uncontrolled bacterial proliferation and severe pathology as well as evidence of complete disruption of the host inflammatory response. When taken in comparison with studies looking at chronic viral infections (42), these observations suggest that PD-1 plays an early homeostatic role in controlling inflammation to foreign antigens (38). Further investigation to delineate the role of PD-1 in controlling inflammation in sarcoidosis pathogenesis is warranted.
A recent report demonstrates that loss of CD4+ T-cell proliferative capacity is present during sarcoidosis disease progression, whereas restoration of CD4+ T-cell function is present with spontaneous clinical resolution (12). The possible correlation of PD-1+ CD4+ T cells with disease progression is likely related to the effects of PD-1 on sarcoidosis proliferative capacity. Investigation of the effects of PD-1 pathway blockade on sarcoidosis CD4+ T-cell proliferative capacity demonstrated restoration of proliferative capacity to healthy control subjects (Figure 6). In granulomatous diseases such as CBD, blockade of the PD-1 pathway is associated with enhanced proliferative capacity (17). Despite PD-1 up-regulation for years in some subjects with sarcoidosis, blockade of the PD-1 pathway resulted in restoration of the sarcoidosis CD4+ T-cell proliferative capacity to a normal range (Figure 6). It has been recently reported that various T-cell effector functions show differential susceptibility to PD-1 signaling. For example, IFN-γ production is relatively resistant to PD-1 engagement, whereas T-cell proliferation and IL-2/tumor necrosis factor-α production are the most sensitive to PD-1 signaling (43). The sensitivity of T-cell expansion to PD-1–mediated inhibition has been attributed to the ability of PD-1 to target early cell-cycle regulators (44, 45). Examination of PD-1 inhibition on CD4+ T-cell proliferation reveals involvement of key regulators of the cell cycle, such as phosphoinositol-3-kinase-AKt (45). Microarray analysis of sarcoidosis PBMC also identified a correlation with the genetic mediators of PD-1 and PI3K expression (Figure 1). Further investigation of the molecular mediators of PD-1–induced inhibition of sarcoidosis proliferative capacity is warranted.
The observation of reduced PD-1 expression with spontaneous clinical resolution and enhanced proliferative capacity with PD-1 pathway blockade, as well as reports of the effects of antiPD-1 or PD-L1 on augmentation of antitumor immunity (46), support consideration of blockade of the PD-1 pathway in sarcoidosis pathogenesis. Clinical trials that target the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers, including non-small cell lung cancer (18, 19, 47). Targeted interventions against this pathway may help restore local and systemic immunity within subjects with sarcoidosis and influence clinical outcome.
Footnotes
Supported by National Institutes of Health grants 1K01HL103179 (K.A.O.-R.) and T32HL069765 (L.J.C.); The Mona Eliassen Foundation; The Pierce Foundation grants U01 HL112694-01, R01 117074, and UL1 RR-024975 (W.P.D.); U01HL112707 (N.K. and W.P.D.); and UO1 CA152662 (P.P.M.). The Vanderbilt University Medical Center Flow Cytometry Shared Resource is supported by Vanderbilt Ingram Cancer Center Grant P30 CA68485 and Vanderbilt Digestive Disease Research Center Grant DK058404.
Author Contributions: Conception and design: W.P.D., K.A.O.-R., and N.K.; performing experiments: N.A.B., L.J.C., J.D.H.-M., S.A., and G.S.; analysis and interpretation: N.A.B., L.J.C., K.A.O.-R., J.D.H.-M., G.S., C.M.S., R.D., J.S., J.G., P.P.M., V.V.P., W.P.D., J.E.J., and D.S.W.; drafting the manuscript for important intellectual content: W.P.D., K.A.O.-R., D.A.C., D.S.W., and N.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201401-0188OC on July 29, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A, Nagy M, Gaede KI, Franke A, Haesler R, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357–364. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 4.Schürmann M, Reichel P, Müller-Myhsok B, Schlaak M, Müller-Quernheim J, Schwinger E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med. 2001;164:840–846. doi: 10.1164/ajrccm.164.5.2007056. [DOI] [PubMed] [Google Scholar]
- 5.Levin AM, Iannuzzi MC, Montgomery CG, Trudeau S, Datta I, McKeigue P, Fischer A, Nebel A, Rybicki BA. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun. 2013;14:13–18. doi: 10.1038/gene.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrianto I, Lin CP, Hale JJ, Levin AM, Datta I, Parker R, Adler A, Kelly JA, Kaufman KM, Lessard CJ, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. PLoS ONE. 2012;7:e43907. doi: 10.1371/journal.pone.0043907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannuzzi MC, Iyengar SK, Gray-McGuire C, Elston RC, Baughman RP, Donohue JF, Hirst K, Judson MA, Kavuru MS, Maliarik MJ, et al. Genome-wide search for sarcoidosis susceptibility genes in African Americans. Genes Immun. 2005;6:509–518. doi: 10.1038/sj.gene.6364235. [DOI] [PubMed] [Google Scholar]
- 8.Crouser ED, Culver DA, Knox KS, Julian MW, Shao G, Abraham S, Liyanarachchi S, Macre JE, Wewers MD, Gavrilin MA, et al. Gene expression profiling identifies MMP-12 and ADAMDEC1 as potential pathogenic mediators of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2009;179:929–938. doi: 10.1164/rccm.200803-490OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS, Ma SF, Wade MS, Noth I, Machado RF, et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS ONE. 2012;7:e44818. doi: 10.1371/journal.pone.0044818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oswald-Richter KA, Richmond BW, Braun NA, Isom J, Abraham S, Taylor TR, Drake JM, Culver DA, Wilkes DS, Drake WP. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J Immunol. 2013;190:5446–5453. doi: 10.4049/jimmunol.1202891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, Fontenot AP. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 2008;180:2704–2712. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 21.Scadding JG. Prognosis of intrathoracic sarcoidosis in England: a review of 136 cases after five years’ observation. Br Med J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, Jenkins CA, Judson MA, Drake WP. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77:3740–3748. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oswald-Richter KA, Beachboard DC, Zhan X, Gaskill CF, Abraham S, Jenkins C, Culver DA, Drake W. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res. 2010;11:161. doi: 10.1186/1465-9921-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutherford RM, Kehren J, Staedtler F, Chibout SD, Egan JJ, Tamm M, Gilmartin JJ, Brutsche MH. Functional genomics in sarcoidosis—reduced or increased apoptosis? Swiss Med Wkly. 2001;131:459–470. doi: 10.4414/smw.2001.09808. [DOI] [PubMed] [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski N, Friedman N. Practical approaches to analyzing results of microarray experiments. Am J Respir Cell Mol Biol. 2002;27:125–132. doi: 10.1165/ajrcmb.27.2.f247. [DOI] [PubMed] [Google Scholar]
- 27.Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richmond BW, Ploetze K, Isom J, Chambers-Harris I, Braun NA, Taylor T, Abraham S, Mageto Y, Culver DA, Oswald-Richter KA, et al. Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-γ expression. J Clin Immunol. 2013;33:446–455. doi: 10.1007/s10875-012-9817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltini C, Spurzem JR, Lee JJ, Pinkston P, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest. 1986;77:1962–1970. doi: 10.1172/JCI112525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- 31.Mou Z, Muleme HM, Liu D, Jia P, Okwor IB, Kuriakose SM, Beverley SM, Uzonna JE. Parasite-derived arginase influences secondary anti-Leishmania immunity by regulating programmed cell death-1-mediated CD4+ T cell exhaustion. J Immunol. 2013;190:3380–3389. doi: 10.4049/jimmunol.1202537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 34.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, Teirstein AS, Zhang Y, Cotter RJ, Moller DR. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald-Richter KA, Beachboard DC, Seeley EH, Abraham S, Shepherd BE, Jenkins CA, Culver DA, Caprioli RM, Drake WP. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol. 2012;32:1129–1140. doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis. 2013;208:603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 38.Henao-Tamayo M, Irwin SM, Shang S, Ordway D, Orme IM. T lymphocyte surface expression of exhaustion markers as biomarkers of the efficacy of chemotherapy for tuberculosis. Tuberculosis (Edinb) 2011;91:308–313. doi: 10.1016/j.tube.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei B, Wang L, Zhao X, Du C, Guo Y, Sun Z. The upregulation of programmed death 1 on peripheral blood T cells of glioma is correlated with disease progression. Tumour Biol. 2014;35:2923–2929. doi: 10.1007/s13277-013-1376-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 41.Lázár-Molnár E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA. 2013;110:E2480–2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11:4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]