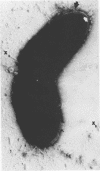
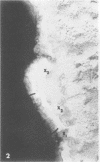
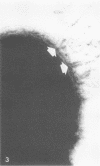
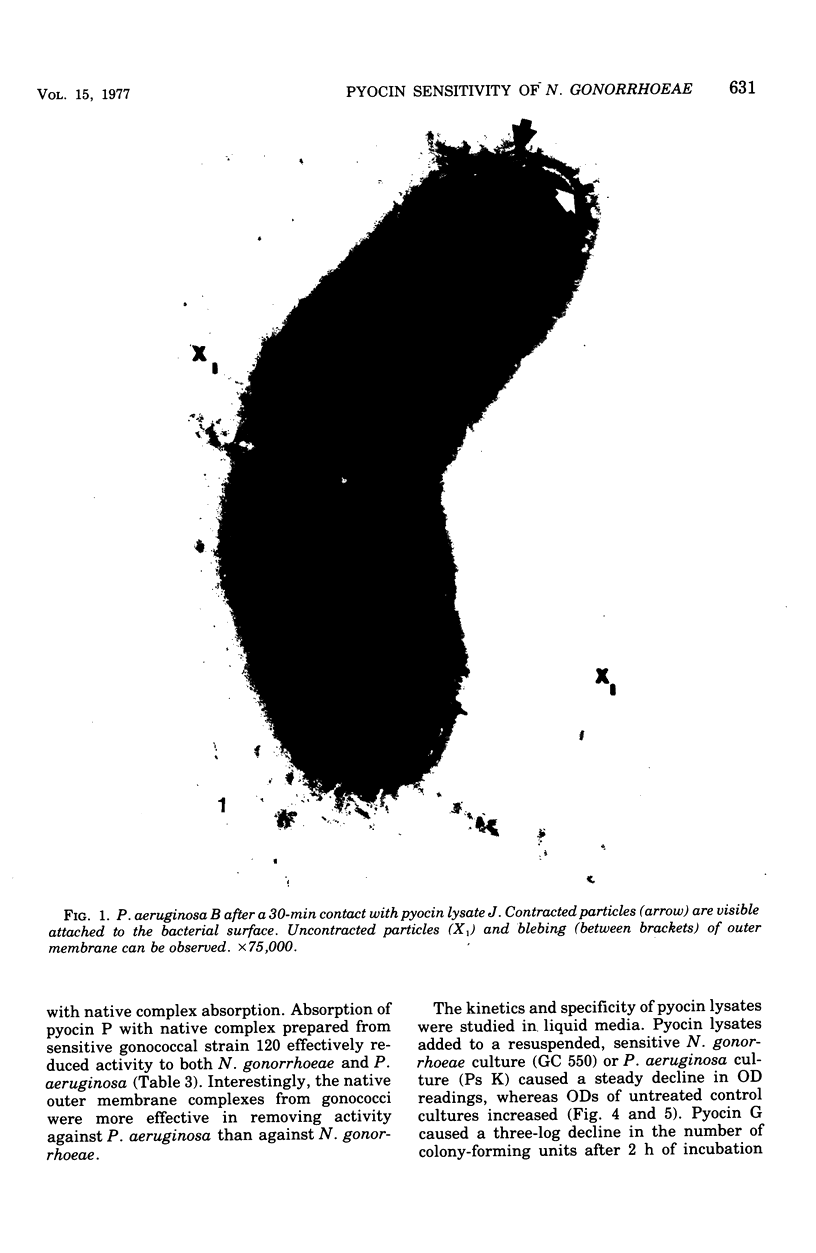
Abstract
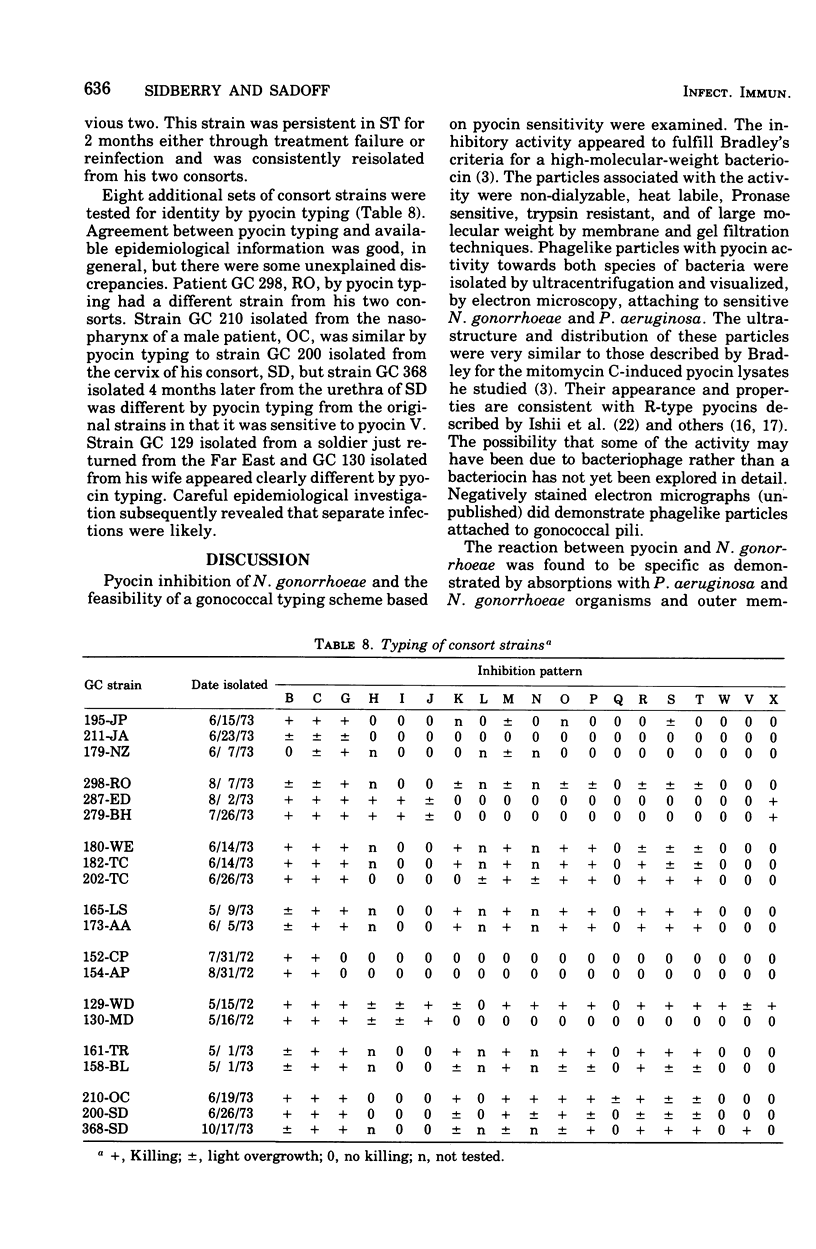
Pyocin inhibition of Neisseria gonorrhoeae and its feasibility as a gonococcal typing scheme were examined. Mitomycin C-induced pyocin lysates of Pseudomonas aeruginosa were able to selectively inhibit the growth of gonococcal strains. The particles associated with the inhibitory activity were non-dialyzable, heat labile, Pronase sensitive, trypsin resistant, and of large molecular weight by membrane and gel filtration techniques. The inhibitory activity was shown to be specific by absorption with sensitive and insensitive strains of N. gonorrhoeae and P. aeruginosa. Partial purification of pyocin lysates by ammonium sulfate precipitation followed by ultracentrifugation revealed phagelike particles consistent with high-molecular-weight R-type pyocines. These particles were associated with increased inhibitory activity and could be seen associated with the gonococcal cell surface. One hundred and six gonococcal strains could be differentiated on the basis of their sensitivity of 23 pyocin extracts. Thirty different patterns of pyocin inhibition were seen. Isolates from different body sites from the same patient could generally be identified as being similar strains. Strains isolated from known consorts had the same patterns. In general, agreement between pyocin typing and available epidemiological information was good.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis. 1974 Dec;130(6):619–625. doi: 10.1093/infdis/130.6.619. [DOI] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Dobek A. S., Stark F. R., Artenstein M. S. Clinical and epidemiological correlates of Pseudomonas typing. J Infect Dis. 1974 Nov;130 (Suppl)(0):S53–S59. doi: 10.1093/infdis/130.supplement.s53. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANARIN I. An investigation of neisseria gonorrhoeae by a red cell sensitization technique. J Hyg (Lond) 1954 Dec;52(4):425–443. doi: 10.1017/s0022172400036901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper W. A. The Serological Classification of Gonococci by Comparative Agglutination. J Bacteriol. 1937 Oct;34(4):353–379. doi: 10.1128/jb.34.4.353-379.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko C. W., Nair G. M. Sero-diagnosis of gonorrhoea with a microprecipitin test using a lipopolysaccharide antigen from N. gonorrhoeae. Br J Vener Dis. 1969 Mar;45(1):33–39. doi: 10.1136/sti.45.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEACON W. E., PEACOCK W. L., Jr, FREEMAN E. M., HARRIS A. Identification of Neisseria gonorrhoeae by means of fluorescent antibodies. Proc Soc Exp Biol Med. 1959 Jun;101(2):322–325. doi: 10.3181/00379727-101-24925. [DOI] [PubMed] [Google Scholar]
- Danielsson D. G., Schmale J. D., Peacock W. L., Jr, Thayer J. D. Antigens of Neisseria gonorrhoeae: characterization by gel filtration, complement fixation, and agar-gel diffusion of antigens of gonococcal protoplasm. J Bacteriol. 1969 Mar;97(3):1012–1017. doi: 10.1128/jb.97.3.1012-1017.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke J., Berk R. S. Growth inhibition and pyocin receptor properties of endotoxin from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1974 Apr;145(4):1405–1408. doi: 10.3181/00379727-145-38023. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J., McEntegart M. G. Bacteriocins from Neisseria gonorrhoeae and their possible role in epidemiological studies. J Clin Pathol. 1972 Jan;25(1):60–61. doi: 10.1136/jcp.25.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geizer E. Antibacterial substances produced by different bacteria inhibiting the growth of Neisseria gonorrhoea (preliminary report). J Hyg Epidemiol Microbiol Immunol. 1968;12(2):241–243. [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974 Jan;80(1):1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Baechler C. A., Berk R. S. In vitro and in vivo characterization of pyocin. J Bacteriol. 1967 Jun;93(6):1976–1986. doi: 10.1128/jb.93.6.1976-1986.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp S. S., Lawton W. D., Chen N. C., Gaafar H. A. Inhibition of Neisseria gonorrhoeae by a factor produced by Candida albicans. Appl Microbiol. 1974 Jan;27(1):192–196. doi: 10.1128/am.27.1.192-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Wiseman G. M. Antibacterial substances from staphylococci. Can J Microbiol. 1967 Aug;13(8):947–955. doi: 10.1139/m67-127. [DOI] [PubMed] [Google Scholar]
- Hutchinson R. I. Typing the gonococcus. Br Med J. 1970 Jul 11;3(5714):107–107. doi: 10.1136/bmj.3.5714.107-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- Kraus S. J., Ellison N. Resistance to gonorrhea possibly mediated by bacterial interference. Appl Microbiol. 1974 Jun;27(6):1014–1016. doi: 10.1128/am.27.6.1014-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Artenstein M. S. The outer cell-wall membrane of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S81–S93. doi: 10.1093/infdis/130.supplement.s81. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Yobs A. R. Colonial morphology of Neisseria gonorrhoeae isolated from males and females. J Bacteriol. 1967 Jan;93(1):513–513. doi: 10.1128/jb.93.1.513-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W., Gray E. D. Group A streptococcal bacteriocin. Production, purification, and mode of action. J Exp Med. 1973 Nov 1;138(5):1168–1183. doi: 10.1084/jem.138.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON J. F. A serological study of Neisseria gonorrhoeae. J Pathol Bacteriol. 1954 Oct;68(2):495–510. doi: 10.1002/path.1700680222. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Kasper D. L., Veltri B. J., Artenstein M. S. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect Immun. 1972 Nov;6(5):835–851. doi: 10.1128/iai.6.5.835-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]