SUMMARY
The dynamic aspects of epilepsy, in which seizures occur sporadically and are interspersed with periods of relatively normal brain function, present special challenges for neuroanatomical studies. While numerous morphological changes can be identified during the chronic period, the relationship of many of these changes to seizure generation and propagation remain unclear. Mossy fiber sprouting is an example of a fequently observed morphological change for which a functional role in epilepsy continues to be debated. This review will focus on neuroanatomically-identified changes that would support high levels of activity in reorganized mossy fibers and potentially associated granule cell activation. Early ultrastructural studies of reorganized mossy fiber terminals in human temporal lobe epilepsy tissue have identified morphological substrates for highly efficacious excitatory connections among granule cells. If similar connections in animal models contribute to seizure activity, activation of granule cells would be expected. Increaed labeling with two activity-related markers, Fos and phosphorylated extracellular signal-regulated kinase, has suggested increased activity of dentate granule cells at the time of a spontaneous seizures in a mouse model of epilepsy. However, neuroanatomical support for a direct link between activation of reorganized mossy fiber terminals and increased granule cell activity remains elusive. As novel activity-related markers are developed, it may yet be possible to demonstrate such functional links and allow mapping of seizure activity throughout the brain. Relating patterns of neuronal activity during seizures to the underlying morphological changes could provide important new insights into the basic mechanisms of epilepsy and seizure generation.
Keywords: Electron microscopy, Extracellular signal-regulated kinase, Fos, Mossy fiber sprouting, Seizure, Temporal lobe epilepsy
Introduction
Neuroanatomical methods have contributed substantially to our understanding of the multiple changes that occur in the epilepsies. From the earliest studies of epilepsy tissue, cell loss has been identified as a significant histopathological change in human tissue (Sommer, 1880; Margerison and Corsellis, 1966), and similar patterns of cell loss have been observed in animal models of acquired epilepsy. Subsequently, with the development of immunohistochemical methods, it became possible to determine the neurochemical identity of many vulnerable neurons (Robbins et al., 1992; Obenaus et al., 1993; Buckmaster and Dudek, 1997; Magloczky and Freund, 2005). This has added valuable functional information, such as identifying a loss of subgroups of GABA neurons, and has suggested ways in which the cell loss could contribute to the pathological process. Immunohistochemical methods have also been important in identifying the myriad of changes that occur in remaining neurons, including the up-regulation and down-regulation of numerous neuropeptides and neurotransmitters, as well as their receptors (Houser and Esclapez, 1996; Baraban and Tallent, 2004; Fritschy, 2008; Sperk et al., 2009, for reviews). Finally, neuroanatomical studies have provided clear demonstrations of the reorganization of axons of several groups of remaining neurons (e.g. Tauck and Nadler, 1985; Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991; Wenzel et al., 2000; Smith and Dudek, 2002; Cavazos et al., 2004; Zhang et al., 2009). All of these findings have played critical roles in the formulation of hypotheses that have guided studies of the basic mechanisms of epilepsy.
Although numerous morphological and neurochemical changes have been identified in epilepsy, many of these changes appear relatively static after they have developed. Yet seizures occur sporadically. Thus the dynamic nature of epilepsy presents special challenges for neuroanatomical studies. How do the persistent neuroanatomical alterations relate to the abnormal, paroxysmal activity that occurs in the brain during seizure activity and where does such activity originate?
To address these questions, a new dimension is needed in our neuroanatomical studies that would allow the identification of activated neurons and circuits. This is an exciting challenge that will be aided greatly by current progress in the identification of novel activity-related markers and the development of unique optical tools for identifying activated neurons and, potentially, altered circuits.
This review will focus on efforts of our laboratory and others to relate the morphological findings in epilepsy to altered function. The ultrastructural features of reorganized mossy fiber terminals in human temporal lobe epilepsy (TLE), as they relate to the possible activity of these terminals, will be considered first. We will then discuss initial studies of activity-related markers that have been used to determine if dentate granule cells, from which the reorganized mossy fibers originate, are activated at the time of a spontaneous seizure in an animal model of recurrent seizures. Detailed descriptions of the methods used in these studies have been provided in the initial reports (Zhang and Houser, 1999; Peng and Houser, 2005; Houser et al., 2008).
Ultrastructure predicts strong activity of reorganized mossy fiber terminals
Mossy fiber sprouting provides a prime example of a dramatic morphological change for which the functional significance continues to be debated. This pattern of axonal reorganization, with a distinct band of aberrant fibers in the inner molecular layer of the dentate gyrus (Fig. 1A) remains one of the most consistent changes in human TLE (Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991; Swartz et al., 2006; Thom et al., 2009) and related animal models. The change in the position of the axon terminals from their concentration in the hilus to the inner molecular layer immediately suggests a major change in circuitry. However, the functional effects also will depend on the subcellular characteristics of the reorganized axon terminals and their postsynaptic targets. The ultrastructural features of the reorganized axon terminals in human TLE are particularly interesting in this regard as they demonstrate changes that occur in the actual disease process and may have developed over many years.
Figure 1.
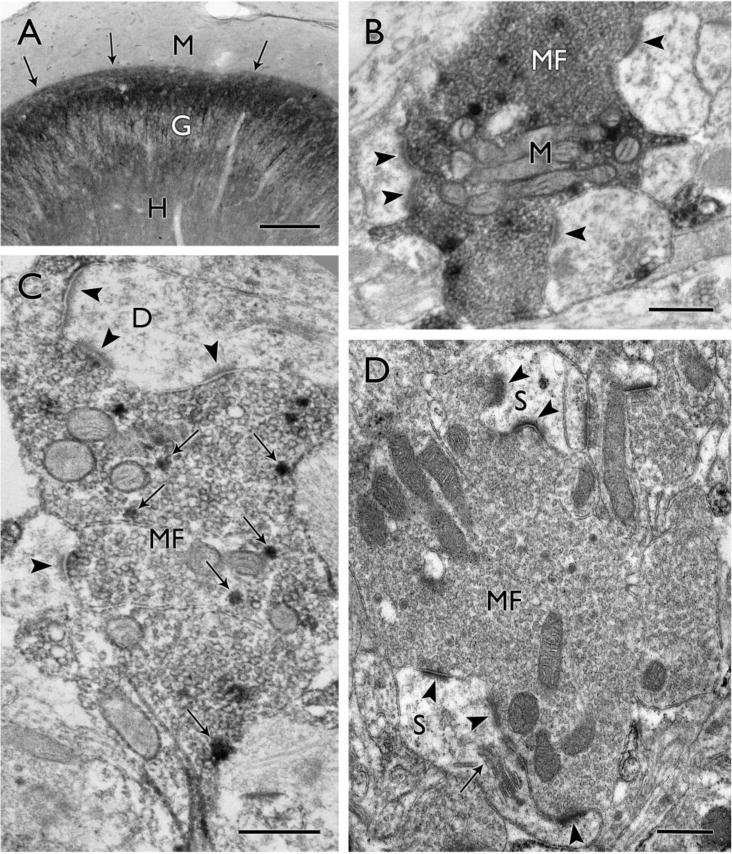
Mossy fiber reorganization in surgical specimens from humans with temporal lobe epilepsy. (A) Dynorphin-labeled mossy fibers form a distinct, aberrant band (arrows) in the inner molecular layer (M), immediately above the granule cell layer (G), while the normally strong dynorphin labeling in the hilus (H) is reduced. (B) A large dynorphin-labeled mossy fiber terminal (MF) contains a cluster of mitochondrial profiles (M) and forms asymmetric synaptic contacts (arrowheads) on at least three different dendritic profiles in this single electron micrograph. (C) In a more lightly-labeled mossy fiber terminal (MF), dynorphin-labeled dense core vesicles (examples at arrows) are scattered among the numerous clear synaptic vesicles that fill the terminal. The terminal forms multiple asymmetric synaptic contacts (arrowheads) on one dendritic profile (D) and an additional synaptic contact on another. (D) A large mossy fiber terminal (MF) is packed with synaptic vesicles and forms synaptic contacts with complex spines (S). In the upper part of the field, two distinct synaptic contacts (arrowheads) are separated by a protrusion from a spine, creating a perforated synapse. In the lower part of the field, multiple synaptic contacts (arrowheads) are formed with a large spine that contains a spine apparatus (arrow). Scale bars = A, 200 μm; B–D, 0.5 μm.
In electron microscopic studies of human tissue, reorganized mossy fibers have been identified by Timm’s staining, intracellular injections of biocytin and immunohistochemical localization of dynorphin (Sutula et al., 1989; Babb et al., 1991; Isokawa et al., 1993; Franck et al., 1995; Zhang and Houser, 1999), and each method has provided unique information. Because dynorphin labeling is concentrated primarily around dense core vesicles it allows a particularly clear view of other features of the terminals, and the abundance of synaptic vesicles is one of the most striking features (Fig. 1B–D). Virtually all labeled terminals are packed with small synaptic vesicles that presumably contain glutamate, the major excitatory neurotransmitter in these terminals. A second critical feature is the presence of numerous asymmetric synaptic contacts (Fig. 1B–D). These ultrastructural features immediately suggest fully developed and functional excitatory connections in this region (Amaral and Dent, 1981). Furthermore, the dense packing of synaptic vesicles adjacent to synaptic contacts, as well as throughout the interior of the terminals, are consistent with a large pool of readily releasable vesicles, as well as a large reserve pool of vesicles, that could support sustained, repetitive firing.
Dense core vesicles that are labeled for dynorphin are distributed throughout the terminals and serve as markers of mossy fiber terminals in this region (Fig. 1C). However, the function of dynorphin within the reorganized mossy fiber pathway remains unclear. While several studies have suggested that dynorphin could mediate anticonvulsant effects (Simmons and Chavkin, 1996; Simonato and Romualdi, 1996; Loacker et al., 2007; Pirker et al., 2009), a recent study suggests that dynorphin, through activation of kappa opioid receptors, could increase the excitability of dentate granule cells (McDermott and Schrader, 2011).
Other ultrastructural features which suggest that reorganized terminals could support strong synaptic activity include a large number of mitochondrial profiles that are either dispersed throughout the mossy fiber terminal or concentrated in clumps among the synaptic vesicles (Fig. 1B). The presynaptic mitochondria would be expected to support the metabolic demands of these terminals. However, they also could provide a local source of calcium that could contribute to short-term enhancement of synaptic activity such as post-tetanic potentiation, as has been described for large mossy fiber terminals in normal rats (Lee et al., 2007). This in turn could support trains of spikes in mossy fiber terminals that appear to be necessary for discharging their postsynaptic targets in normal animals (Henze et al., 2002).
The majority of synaptic contacts of the reorganized mossy fibers were with either dendritic spines or dendritic shafts (Fig. 1C,D), and in some instances the postsynaptic element was also labeled with dynorphin. These findings suggest that the postsynaptic targets are granule cells. Granule cell to granule cell contacts have also been described in other studies of human epilepsy tissue as well as most animal models (Represa et al., 1993; Franck et al., 1995; Okazaki et al., 1995; Buckmaster et al., 2002; but also see Sloviter et al., 2006).
As further evidence for a strongly interconnected excitatory circuit, many of the reorganized mossy fiber terminals formed multiple synapses within a single section (Fig. 1C,D). In some instances, the synapses were formed with several different postsynaptic profiles (Fig. 1B–D). It is not known whether the different postsynaptic profiles belong to the same or different granule cells, but contacts on several different postsynaptic neurons, as suggested in several studies of animal models (Okazaki et al., 1995; Buckmaster et al., 2002), could enhance synchronous firing among the granule cells. In addition, multiple synaptic contacts were often formed on the same postsynaptic profile (Fig. 1C,D), and such connectivity would be expected to have particularly strong influences on the postsynaptic targets.
One could speculate that ongoing seizure activity, if it were to engage the reorganized mossy fibers, could contribute to the remodeling of the presynaptic terminals as well as the postsynaptic spines. Indeed, recent studies demonstrate that mossy fiber terminals can undergo considerable growth and remodeling even in normal adult mice in response to spiking activity and an enriched environment, and these changes could, in turn, increase their synaptic strength (Galimberti et al., 2006). The ultrastructural features of mossy fiber sprouting are particularly interesting when viewed from this perspective.
The large size of many of the reorganized mossy fiber terminals is one of the most unique and striking features of these terminals in human TLE (Fig. 1B–D), and would be consistent with their involvement in an active circuit. Single profiles as large as 6.2 μm in major diameter were identified, with a mean of 2.3 μm (Zhang and Houser, 1999). Thus these reorganized terminals approach the size of normal large mossy fiber terminals that contact mossy cells in the hilus and CA3 pyramidal cells. This contrasts with some findings in rodent models in which most mossy fiber terminals were small to moderate in size (Okazaki et al., 1995; Cavazos et al., 2003). This difference could be related to the more extended time course of epilepsy in humans (years) compared to that in most animals models (weeks to months).
The spines that are contacted by the large reorganized mossy fibers may also display activity-related changes. In the human tissue, the large mossy fiber terminals often formed synaptic contacts with spines that extended into the mossy fiber terminals and appeared to be complex spines (Zhang and Houser, 1999). Similar complex spines in contact with reorganized mossy fibers have also been observed in the alumina gel model of TLE in monkeys (Ribak et al., 1998). While complex spines or thorny excrescences are prominent postsynaptic targets of mossy fiber terminals as they extend from mossy cells in the hilus or pyramidal cell dendrites in CA3, such spines are generally not found on normal dentate granule cells in the inner molecular layer. Thus it is possible that complex spines could have been induced by the large reorganized mossy fibers (Represa et al., 1993; Ribak et al., 1998)
Multiple synaptic contacts were commonly observed on the large spines, and many are likely to be perforated synapses (Fig. 1D). Such synapses consist of distinct active zones with thickened postsynaptic densities that are separated by a short segment of plasma membrane which lacks a postsynaptic thickening. The synaptic contacts were often separated by an extension from the spine that could contribute to more complete functional separation of the active zones (Fig. 1D), and thus could increase synaptic strength (Edwards, 1995). Such perforated synapses are considered to be highly active and are increased in the dentate gyrus following kindling and long term potentiation (Geinisman et al., 1990; Geinisman et al., 1991). Interestingly, increased expression of some glutamatergic AMPA receptors have been observed at perforated synapses, compared to non-perforated synapses, in CA1 pyramidal cell dendrites in normal animals (Ganeshina et al., 2004). Furthermore, in AMPA glutamate receptor 2 (GluR2) knockout mice, the proportion of synapses with perforated post-synaptic densities was significantly decreased in the dentate gyrus, supporting the suggestion that GluR2 AMPA receptors have a significant influence on synapse and spine architecture (Medvedev et al., 2008). Since increased AMPA receptor expression has been observed in the molecular layer in humans with TLE and some animal models (Babb et al., 1996; Lynd-Balta et al., 1996; Lynd-Balta et al., 2004), it would be quite interesting to find that AMPA receptors are enriched at the perforated synapses that are postsynaptic to reorganized mossy fiber terminals in the epilepsy tissue.
Studies in animal models provide additional information on the patterns of reorganization of mossy fibers, and confirm the formation of numerous direct, monosynaptic excitatory connections among granule cells, in contrast to the virtual lack of connectivity among granule cells in normal animals. In addition, the rostral-caudal projections of reorganized mossy fibers are expanded in the epileptic animals (Buckmaster and Dudek, 1999), contrasting with the lamellar organization of normal mossy fibers (Amaral and Witter, 1989).
These numerous changes provide the morphological substrates for a highly interconnected network of granule cells that could contribute to hyperexcitability and an increase in synchronous firing of these neurons (Nadler, 2003; Sutula and Dudek, 2007, for reviews). However, in many electrophysiological studies, additional experimental manipulations that either decrease inhibition or increase excitation are generally required to elicit epileptiform activity in granule cells in many animal models of epilepsy. Whether such changes occur spontaneously in vivo remains unknown, but it is possible that periodic decreases in inhibition or increases in extracellular potassium or excitatory input, could lead to activation of granule cells and, through the reorganized mossy fiber terminals, to increased activity throughout large regions of the dentate gyrus.
Indeed, if reorganized mossy fibers play a role in seizure activity, then activation of granule cells would be expected to occur at the time of a spontaneous seizure. However, since numerous changes, in addition to mossy fiber sprouting, occur in dentate granule cells in epilepsy, increased granule cell firing is not solely or necessarily related to mossy fiber sprouting. Nevertheless, if mossy fiber sprouting is critically involved in the seizure process, granule cell activity should be detected. In an effort to map granule cell activity in mice with recurrent seizures, we used activity-related markers to determine if granule cells are activated at early time points after detection of a spontaneous seizure.
Fos expression suggests granule cell activity in association with spontaneous seizures
Expression of the immediate early gene c-fos and the protein Fos has been used in many experimental paradigms, including induced seizures, to demonstrate activated neurons. However, only a few studies have examined Fos expression after spontaneous seizures, and the results have differed (Harvey and Sloviter, 2005; Mello et al., 1996; Peng and Houser, 2005). These differences have highlighted several interesting questions regarding granule cell activity. In our studies of a mouse model of epilepsy, light Fos labeling of numerous granule cells was evident throughout the dentate gyrus as early as 15 minutes after detection of a behavioral seizure (Fig. 2A,D). Furthermore, Fos labeling of granule cells was evident prior to strong labeling of GABA neurons in the dentate gyrus (Fig. 2A,B,D,E). Both parvalbumin interneurons along the base of the granule cell layer (Fig. 2C) and GABAergic interneurons in the dentate molecular layer (Fig. 2F) expressed Fos at later time points than granule cells in the same region, consistent with early preferential activation of granule cells and a delay in strong activation of the interneurons (Peng and Houser, 2005).
Figure 2.
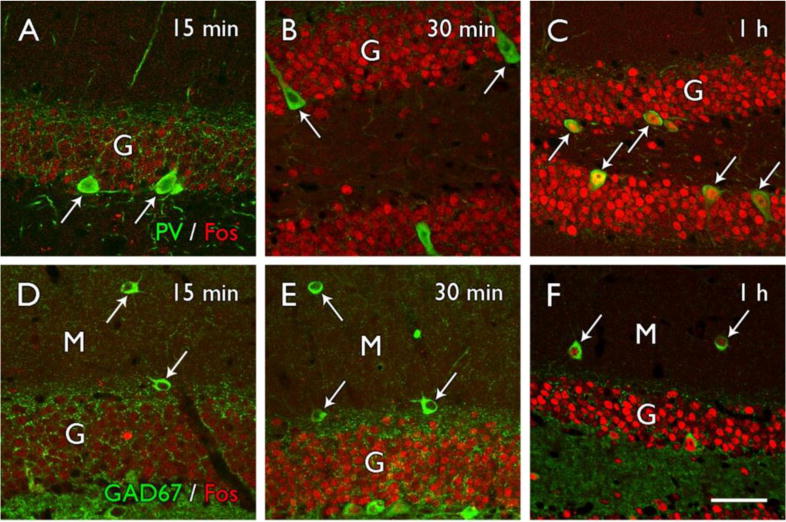
Fos labeling in granule cells precedes that in interneurons of the dentate gyrus following a spontaneous seizure in a mouse model of epilepsy. (A–C) Fos labeling in granule cells (G) is evident as early as 15 minutes and increases to high levels by 30 minutes after a spontaneous seizure. In contrast, parvalbumin-labeled interneurons (arrows) along the base of the granule cell layer remain relatively unlabeled for Fos at 15–30 minutes after a spontaneous seizure but are distinctly Fos-labeled at 1 hour after a seizure. (D–F) GAD67-labeled interneurons (arrows) in the molecular layer (M) of the dentate gyrus show a similar progression in Fos labeling and exhibit strong labeling at 1 hour after a spontaneous seizure but little labeling at earlier time points. Scale bar = A–F, 25 μm. Adapted from Figure 8, J. Neurosci. 25:7210–7220, 2005, with permission.
These findings differ from other reports in which little granule cell labeling was observed at one hour after a spontaneous seizure, despite strong labeling of interneurons (Harvey and Sloviter, 2005). These results have led to the view that granule cells are not highly active at the onset of spontaneous seizures and may, instead, be hyperinhibited by local interneurons (Harvey and Sloviter, 2005; Sloviter et al., 2006). The differences in the basic findings could result from use of different models and different species (mouse versus rat), and also could be influenced by the time at which the Fos labeling was examined, as early as 15 minutes in the mouse model and one hour in the rat.
Electrophysiological studies of granule cell activity at the time of spontaneous seizures have also yielded different results. In one set of studies, synchronous granule cell discharges were not detected before spontaneous seizures in pilocarpine-treated rats during the chronic period (Harvey and Sloviter, 2005). However, in another study in rat, a sizeable fraction of recorded granule cells (63%) became active either before or during a spontaneous seizure (Bower and Buckmaster, 2008). These latter results are consistent with our findings of strong labeling of granule cells in a mouse model of epilepsy at short intervals following detection of a spontaneous seizure. While the electrophysiological changes that underlie Fos expression are complex and remain incompletely understood (Labiner et al., 1993), we have interpreted the Fos labeling in granule cells as an indication that they are active and presumably firing action potentials near the onset of spontaneous seizures. However, we have continued to search for other markers that would have a more rapid time course, allowing us to detect granule cell activation at shorter intervals.
Extracellular signal-regulated kinase activation signals altered neuronal activity in epilepsy
Extracellular signal-regulated kinase (ERK) is part of a mitogen-activated protein kinase (MAPK) signaling pathway that is activated in response to numerous types of stimuli and is involved in several forms of synaptic plasticity, as well as regulation of neuronal excitability (Sweatt, 2004; Thomas and Huganir, 2004, for reviews). Because ERK is activated by phosphorylation, the process is rapid, providing better temporal resolution and possibly allowing detection of a sequence of neuronal activation. While our initial goal was to identify activated neurons at the time of a spontaneous seizure, a change in basal pERK labeling was also found in the seizure-prone mice (Houser et al., 2008). Thus two distinctly different types of changes were signaled by the pERK labeling, representing different points on the continuum of activity-related changes in an epilepsy model.
In normal and control animals, the basal pattern of pERK labeling is characterized by scattered neurons with strong labeling of their cell bodies and dendrites throughout most regions of the forebrain (Figs. 3A–D; 4A). In addition, light to moderate diffuse labeling is evident throughout most dendritic regions, with laminar patterns in some regions (Fig. 4A). The neuronal activity that is associated with the strong labeling in scattered neurons is incompletely understood. Depolarization and action potential firing at low frequencies do not appear to be sufficient (Dudek and Fields, 2001), and it is likely that the strong labeling of selected neurons reflects bursts of action potentials or possibly complex firing patterns that could be information-rich and occur in only limited numbers of neurons (Lisman, 1997; Thomas et al., 1998). However, strong stimulation, including induced seizure activity, increases pERK labeling in large populations of neurons, consistent with strong firing of the neurons (Berkeley et al., 2002; Zhao et al., 2005; Chotiner et al., 2010).
Figure 3.
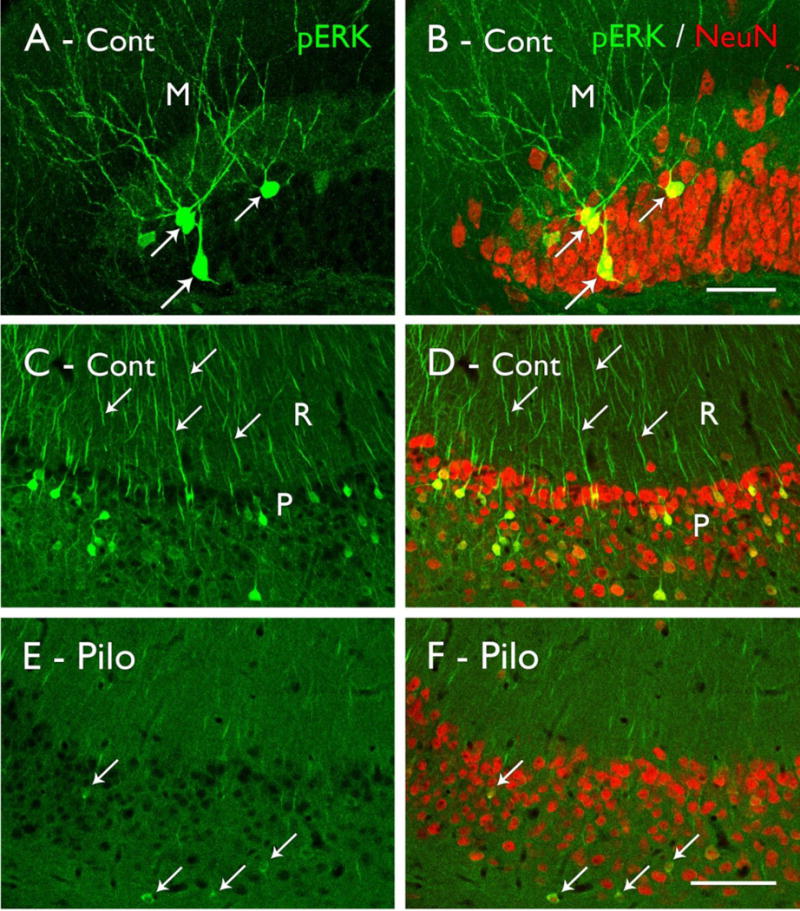
Phosphorylated ERK (pERK) labeling in control (Cont; A–D) and pilocarpine-treated (Pilo; E,F) mice. (A,B) Strong pERK labeling is evident in scattered granule cells in the dentate gyrus (arrows), and their labeled dendrites extend into the molecular layer (M). Double labeling with NeuN demonstrates the relatively small proportion of granule cells that are strongly labeled for pERK in control animals. (C,D) Strong pERK labeling is also evident in selected neurons in the pyramidal cell layer (P) of CA1, and their apical dendrites (examples at arrows) extend into s. radiatum (R). Double labeling with NeuN demonstrates that pERK-labeled neurons are distributed throughout the pyramidal cell layer. (E,F) In a pilocarpine-treated mouse, the numbers of neurons that are strongly labeled for pERK are substantially reduced, although faint labeling can be detected in some neurons (arrows). Double-labeling with NeuN demonstrates that the reduced labeling is not due to cell loss in the pyramidal cell layer in this animal. (Scale bars = A,B, 20 μm; C–F, 100 μm.
Figure 4.
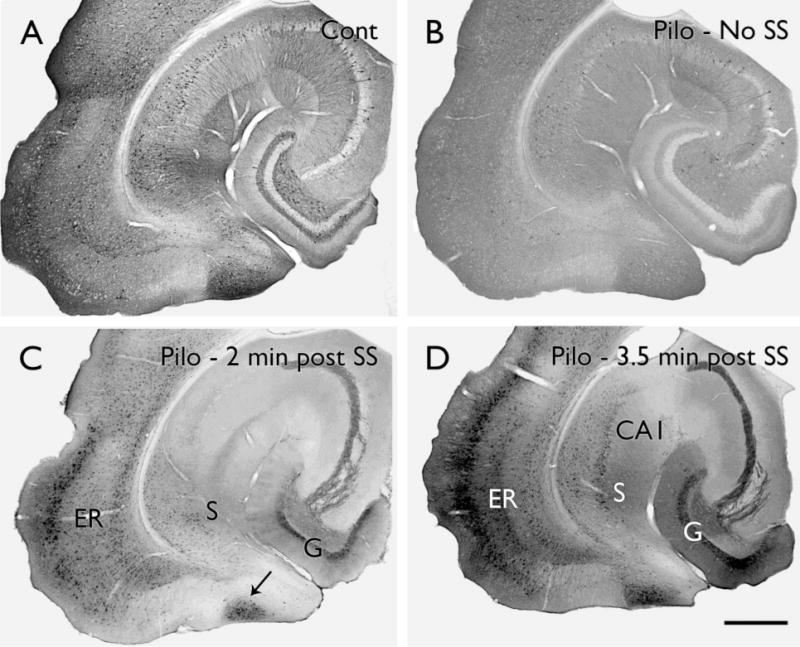
Alterations in phosphorylated ERK labeling in pilocarpine-treated mice. (A) In a control (Cont) mouse, scattered, strongly-labeled neurons are evident in most brain regions and stand out against a background of diffuse pERK labeling. (B) In a pilocarpine (Pilo)-treated mouse that had not experienced a spontaneous seizure (SS) during the previous 24 hours, the number of neurons with strong pERK labeling is substantially decreased throughout much of the hippocampal formation and associated limbic cortex. (C) In a pilocarpine-treated mouse at approximately 2 minutes after detection of a spontaneous behavioral seizure, pERK labeling is strongly increased in several limbic regions, including a large extent of the dentate granule cell layer (G), lateral entorhinal cortex (ER), subiculum (S) and a unique zone (arrow) that is located between the parasubiculum and medial entorhinal cortex. (D) In a mouse at 3.5 minutes after a spontaneous seizure, pERK labeling is substantially increased and is strong throughout the granule cell layer, lateral entorhinal cortex, CA1 and subiculum. Scale bar = A–D, 200 μm.
We thus anticipated increased levels of pERK labeling in our pilocarpine-treated mice during the chronic period when these mice are prone to seizures. Instead, we observed a decrease in the number of strongly-labeled neurons throughout broad regions of the limbic system, including the dentate gyrus, when compared to that in control animals (Fig. 3E,F; 4B). This decreased labeling is reminiscent of the hypometabolism that is often observed throughout limbic regions of humans with TLE, extending beyond regions with cell loss (Engel, Jr. et al., 1982; O’Brien et al., 1997). The functional consequences of the decreases in pERK-labeled neurons remain unknown, but the consistency of these findings and the wide distribution of the changes suggest that they represent a fundamental change in some activity-related processes in the epileptic animals. The decreases in pERK labeling are not related solely to cell loss as they are observed in numerous regions in which the neurons are preserved (Fig. 3E,F). The broad decrease in numbers of distinctly-labeled neurons could reflect decreases in some patterns of network activity, possibly as a compensatory mechanism to counter increased excitability of the system. The changes also could be related to impairments in learning and memory in these animals, as the ERK pathway is critical for these processes (Satoh et al., 2007).
At the time of spontaneous seizures, the picture changed dramatically. Strong pERK labeling could be detected in granule cells of the dentate gyrus, as well as other limbic regions, at very short intervals after detection of a spontaneous seizure (Fig. 4C,D). The neuronal labeling extended throughout the dendrites as well as cell bodies. While the time course of these changes is still being determined, the increase in pERK labeling was evident by two minutes (Fig. 4C) or slightly earlier following detection of a behavioral seizure. The labeling continued to increase (Fig. 4D), reaching maximal levels by six to seven minutes, and then decreased in dentate granule cells by 15 minutes after seizure detection. Interestingly, pERK labeling in granule cells had decreased substantially by the time that Fos labeling was first being detected in these neurons (Houser et al., 2008).
Other in vivo and in vitro studies in which ERK was activated by direct stimulation have also demonstrated that ERK labeling occurs rapidly following strong stimulation and could first be observed at approximately two minutes following the stimulation (Komiyama et al., 2002; Chotiner et al., 2010). This suggests that granule cell activation in the mouse model was occurring at an early stage of the spontaneous seizure, or potentially preceding the seizure. This would be consistent with electrophysiological findings in rats in which granule cell activity preceded the behavioral and electrophysiological seizure onset in some recordings (Bower and Buckmaster, 2008).
Despite limitations in temporal resolution, a major advantage of neuroanatomical studies of activity-related markers is the ability to study activation patterns at multiple levels of the brain in the same animal. Analysis of labeling patterns throughout the rostral-caudal extent of the hippocampal formation could be particularly informative. While the number of animals remains limited, current results suggest that an increase in granule cell labeling does not occur simultaneously throughout the dentate gyrus. Instead, in some animals at the shortest post-seizure intervals, labeling was preferentially observed in the caudal (ventral) regions (Fig. 4C) and only later was granule cell labeling evident in more rostral (dorsal) regions. Also, at short post-seizure intervals, pERK labeling was often stronger in some regions of the granule cell layer than others, even within a given section (Fig. 4C). At slightly longer post-seizure intervals, the granule cell and molecular layers were generally labeled in all regions within the section (Fig. 4D) and throughout their rostral-caudal extent. Such patterns suggest initial increased granule cell activation in limited regions of the dentate gyrus with subsequent expansion of activity throughout the remainder of the population. Such a sequence would be consistent with progressive recruitment of granule cells through mossy fiber sprouting or other mechanisms. With such regional differences in labeling, a lack of granule cell firing might be expected in some regions at the onset of seizure activity, particularly in the dorsal dentate gyrus where electrophysiological recordings are often obtained.
Studies of additional activity-related markers are expected to provide further insights into the patterns of neuronal activation at the time of spontaneous seizures, and potentially identify brain regions with the earliest activity. As methods that label activated axon terminals are discovered, it may yet be possible to provide direct demonstrations of the activation of reorganized mossy fibers and determine the effects on granule cell excitability.
Considerations in use of activity-related markers in epilepsy
While our studies have focused on specific questions regarding activation of granule cells, it will be important to address broader questions in the future, including the sequence of neuronal activation during seizure initiation. The results and their interpretations will be influenced greatly by the characteristics of the activity-dependent markers themselves, as well as the animal models, the associated patterns of cell loss, and other morphological changes on which the patterns of activated neurons and circuits are superimposed.
Each activity-related marker is likely to have unique characteristics that will influence the labeling patterns. Even for current, extensively-used methods, such as Fos and Arc/Arg 3.1 expression, the electrophysiological changes that underlie the activation and labeling are incompletely understood. Yet such information is critical for interpreting the activation patterns in epilepsy. Equally important, the activity-related markers may be restricted to specific cell types. For example, pERK may have different thresholds for activation in different cell types and generally shows very limited expression in many inhibitory interneurons (Sindreu et al., 2007; Houser et al., 2008). Thus, with current methods, pERK labeling cannot be used to identify the sequence of principal cell and interneuron activation. Likewise, the time course of activation of each marker may differ and could substantially influence interpretation of the results. For a marker with a rapid and short activation cycle, analysis at later time points could fail to detect neuronal labeling that occurred earlier but had decreased due to the rapid time course of expression. While such variations may initially complicate the picture, they could eventually be important for identifying different signaling pathways in different subclasses of neurons. Ultimately, such knowledge could contribute to cell-type specific treatments for the prevention and amelioration of epilepsy.
As with electrophysiological studies, the patterns of neuronal activation may differ with different models and times of analysis. Indeed, increasing evidence suggests that the patterns of elicited or spontaneous seizure activity may change over time. Patterns of neuronal activity that are evident at early stages following the initial insult may differ from those that are elicited during the chronic period, possibly related to compensatory changes within the system (Ang et al., 2006; Sloviter et al., 2006; Pathak et al., 2007).
Likewise, differences in the selected animal models, species and strains may influence the patterns of neuronal activation at the time of seizure generation. For example, epilepsy models with large amounts of extra-hippocampal damage may have different sites of seizure activation than those with more restricted damage, despite similar extents of hippocampal cell loss and mossy fiber sprouting. Recognition of such differences and analyses of the neuronal activation patterns, in conjunction with the underlying morphological changes, could provide fresh insights into the general mechanisms of epilepsy.
Conclusions
Relating structure to function has been an important research goal in the epilepsy field. Identification and use of novel markers of neuronal activity could lead to a better understanding of the functional significance of the underlying morphological changes. Such studies could also prove useful in the development of seizure prediction methods that are aimed at halting the progression of seizure activity through site and cell-type specific interventions.
Acknowledgments
This work was supported by Veterans Affairs Medical Research Funds (C.R.H.) and the National Institutes of Health grants NS046524 and NS051311 (C.R.H.). We are grateful to Drs. J.R. Rich, P.S. Dwan, B.E. Swartz, G.O. Walsh and A.V. Delgado-Escueta, and to their patients, for invaluable contributions to our early studies of human temporal lobe epilepsy tissue.
This review is contributed as a tribute to H. Jürgen Wenzel in recognition of his careful, detailed morphological studies which have been linked consistently to function, and his great interest in mossy fibers of the dentate gyrus, from their early development (Wenzel et al., 1981), to their functionally associated transporters (Wenzel et al., 1997), and to their reorganization in epilepsy (Wenzel et al., 2000).
Footnotes
DISCLOSURE
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with these guidelines.
References
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Babb TL, Mathern GW, Leite JP, Pretorius JK, Yeoman KM, Kuhlman PA. Glutamate AMPA receptors in the fascia dentata of human and kainate rat hippocampal epilepsy. Epilepsy Res. 1996;26:193–205. doi: 10.1016/s0920-1211(96)00053-8. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides–endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Bower MR, Buckmaster PS. Changes in granule cell firing rates precede locally recorded spontaneous seizures by minutes in an animal model of temporal lobe epilepsy. J Neurophysiol. 2008;99:2431–2442. doi: 10.1152/jn.01369.2007. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Jones SM, Cross DJ. Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience. 2004;126:677–688. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol. 2003;458:272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- Chotiner JK, Nielson J, Farris S, Lewandowski G, Huang F, Banos K, de Leon R, Steward O. Assessment of the role of MAP kinase in mediating activity-dependent transcriptional activation of the immediate early gene Arc/Arg3.1 in the dentate gyrus in vivo. Learn Mem. 2010;17:117–129. doi: 10.1101/lm.1585910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Fields RD. Mitogen-activated protein kinase/extracellular signal-regulated kinase activation in somatodendritic compartments: roles of action potentials, frequency, and mode of calcium entry. J Neurosci. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-02-j0002.2001. RC122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA. Anatomy and electrophysiology of fast central synapses lead to a structural model for long-term potentiation. Physiological Reviews. 1995;75:759–787. doi: 10.1152/physrev.1995.75.4.759. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Kuhl DE, Phelps ME, Mazziotta JC. Interictal cerebral glucose metabolism in partial epilepsy and its relation to EEG changes. Ann Neurol. 1982;12:510–517. doi: 10.1002/ana.410120603. [DOI] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–558. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I balance and GABAA receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti I, Gogolla N, Alberi S, Santos AF, Muller D, Caroni P. Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experience. Neuron. 2006;50:749–763. doi: 10.1016/j.neuron.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004;125:615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566:77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Morrell F, deToledo-Morrell L. Increase in the relative proportion of perforated axospinous synapses following hippocampal kindling is specific for the synaptic field of stimulated axons. Brain Res. 1990;507:325–331. doi: 10.1016/0006-8993(90)90291-i. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF, Babb TL, Engel J. Single mossy fiber axonal systems of human dentate granule cells studied in hippocampal slices from patients with temporal lobe epilepsy. J Neurosci. 1993;13:1511–1522. doi: 10.1523/JNEUROSCI.13-04-01511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O’Carroll CM, Martin SJ, Morris RG, O’Dell TJ, Grant SG. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiner DM, Butler LS, Cao Z, Hosford DA, Shin C, McNamara JO. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J Neurosci. 1993;13:744–751. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lee KH, Ho WK, Lee SH. Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J Neurosci. 2007;27:13603–13613. doi: 10.1523/JNEUROSCI.3985-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130:1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Pilcher WH, Joseph SA. Distribution of AMPA receptor subunits in the hippocampal formation of temporal lobe epilepsy patients. Neuroscience. 1996;72:15–29. doi: 10.1016/0306-4522(95)00554-4. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Pilcher WH, Joseph SA. AMPA receptor alterations precede mossy fiber sprouting in young children with temporal lobe epilepsy. Neuroscience. 2004;126:105–114. doi: 10.1016/j.neuroscience.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes: A clinical encephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- McDermott CM, Schrader LA. Activation of kappa opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J Physiol. 2011;589:3517–3532. doi: 10.1113/jphysiol.2011.211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev NI, Rodriguez-Arellano JJ, Popov VI, Davies HA, Tigaret CM, Schoepfer R, Stewart MG. The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus. Eur J Neurosci. 2008;27:315–325. doi: 10.1111/j.1460-9568.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- Mello LE, Kohman CM, Tan AM, Cavalheiro EA, Finch DM. Lack of Fos-like immunoreactivity after spontaneous seizures or reinduction of status epilepticus by pilocarpine in rats. Neurosci Lett. 1996;208:133–137. doi: 10.1016/0304-3940(96)12562-3. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, Newton MR, Cook MJ, Berlangieri SU, Kilpatrick C, Morris K, Berkovic SF. Hippocampal atrophy is not a major determinant of regional hypometabolism in temporal lobe epilepsy. Epilepsia. 1997;38:74–80. doi: 10.1111/j.1528-1157.1997.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Gasser E, Czech T, Baumgartner C, Schuh E, Feucht M, Novak K, Zimprich F, Sperk G. Dynamic up-regulation of prodynorphin transcription in temporal lobe epilepsy. Hippocampus. 2009;19:1051–1054. doi: 10.1002/hipo.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Jorquera I, Le Gal La Salle G, Ben-Ari Y. Epilepsy induced collateral sprouting of hippocampal mossy fibers: does it induce the development of ectopic synapses with granule cell dendrites? Hippocampus. 1993;3:257–268. doi: 10.1002/hipo.450030303. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Weber P, Epstein CM, Henry TR, Bakay RA. Alumina gel injections into the temporal lobe of rhesus monkeys cause complex partial seizures and morphological changes found in human temporal lobe epilepsy. J Comp Neurol. 1998;401:266–290. [PubMed] [Google Scholar]
- Robbins RJ, Brines ML, Kim JH, Adrian T, de Lanerolle NC, Welsh S, Spencer DD. A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 1992;29:325–332. doi: 10.1002/ana.410290316. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Simonato M, Romualdi P. Dynorphin and epilepsy. Prog Neurobiol. 1996;50:557–583. doi: 10.1016/s0301-0082(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Dudek FE. Network interactions mediated by new excitatory connections between CA1 pyramidal cells in rats with kainate-induced epilepsy. J Neurophysiol. 2002;87:1655–1658. doi: 10.1152/jn.00581.2001. [DOI] [PubMed] [Google Scholar]
- Sommer W. Erkrankung des Ammonshorns als aetiologisches moment der epilepsie. Arch Psychiatr Nervenkr. 1880;10:631–675. [Google Scholar]
- Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in post-traumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Martinian L, Catarino C, Yogarajah M, Koepp MJ, Caboclo L, Sisodiya SM. Bilateral reorganization of the dentate gyrus in hippocampal sclerosis: a postmortem study. Neurology. 2009;73:1033–1040. doi: 10.1212/WNL.0b013e3181b99a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O’Dell TJ. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Woolley CS, Robbins CA, Schwartzkroin PA. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10:244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Stender G, Duwe G. Development of neuron structure of the fascia dentata in the rat. Neurohistologico-morphometric, ultrastructural and experimental study. J Hirnforsch. 1981;22:629–683. [PubMed] [Google Scholar]
- Zhang N, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J Comp Neurol. 1999;405:472–490. [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Adams JP, Dudek SM. Pattern-dependent role of NMDA receptors in action potential generation: consequences on extracellular signal-regulated kinase activation. J Neurosci. 2005;25:7032–7039. doi: 10.1523/JNEUROSCI.1579-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


