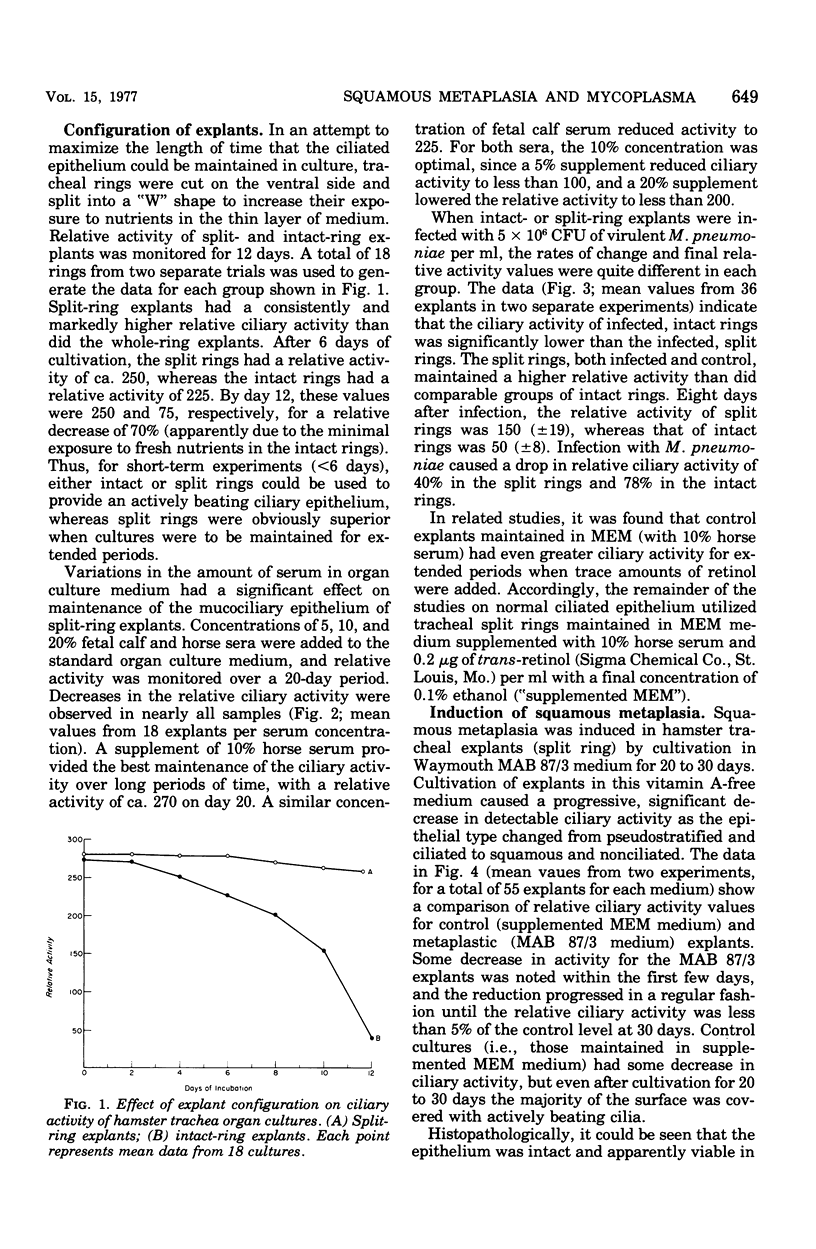
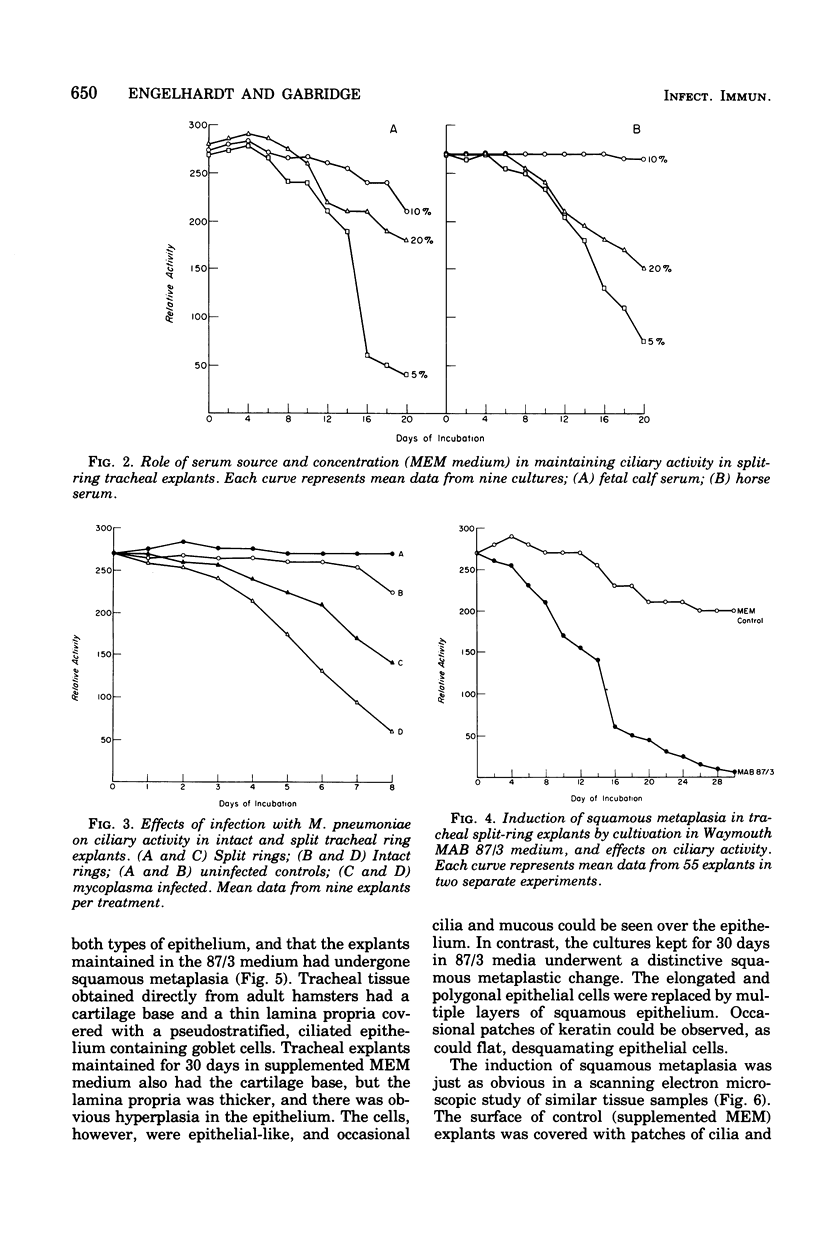
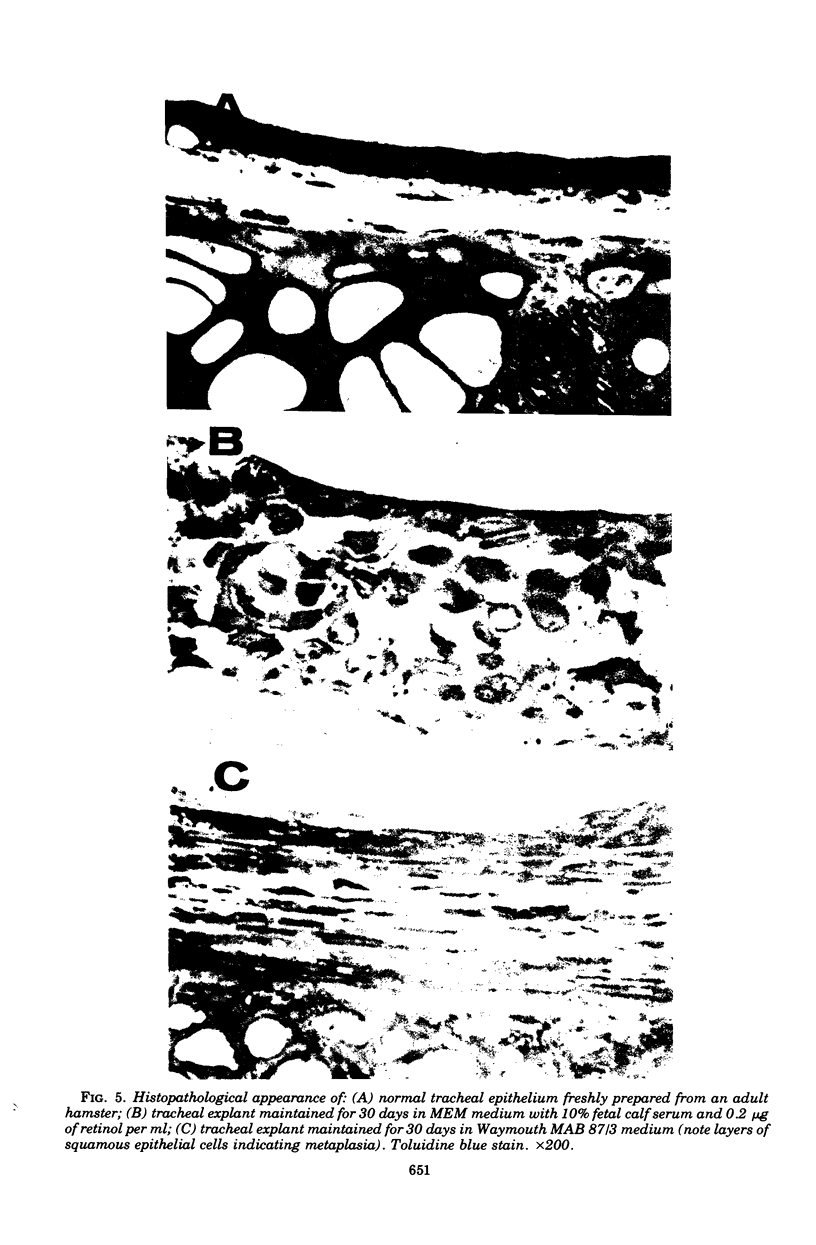
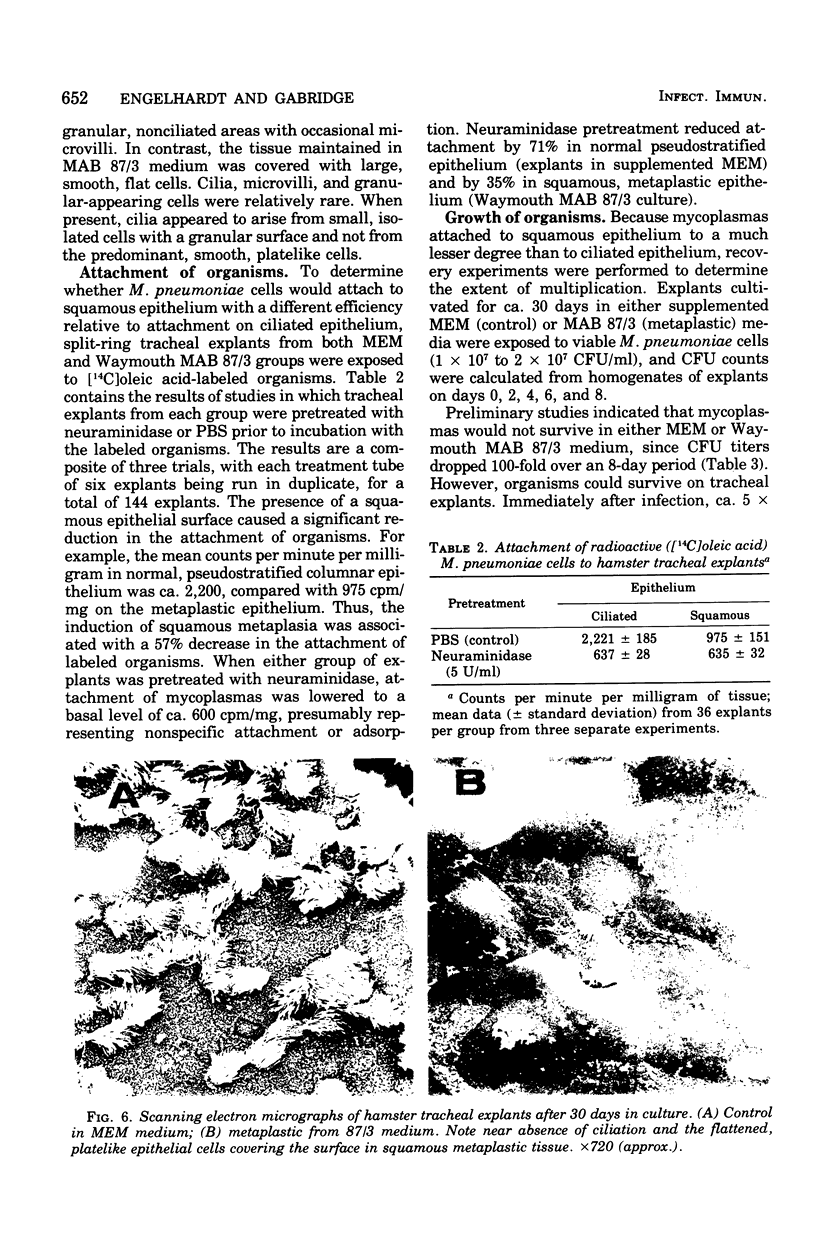
Abstract
An organ culture system for hamster trachea was developed for maintenance of the ciliated respiratory epithelium during periods of extended cultivation (i.e., greater than 20 days). Evaluation of five serum types showed that horse serum and fetal calf serum were best for the maintenance of epithelial ciliary activity and morphology. Rings that were opened on one side ("split rings") had the best maintenance of the ciliated epithelium as judged by the retention of ciliary activity and normal histological appearance after 3 to 4 weeks in culture. The in vitro induction of squamous metaplasia was achieved by cultivating explants in Waymouth MAB 87/3 (vitamin A-free) medium, without serum. This system allowed a direct comparison of the effects of Mycoplasma pneumoniae infection in two epithelial types, ciliated pseudostratified columnar and keratinizing squamous. Attachment of 14C-labeled mycoplasmas was more than twofold greater in the normal epithelium. Pretreatment of explants with neuraminidase decreased attachment for both squamous and pseudostratified epithelial surfaces to a similar basal level. Recovery of viable organisms from infected tissue of both epithelial types indicated that the organism titer remained essentially constant during the infection period, but was significantly higher for the pseudostratified ciliated epithelium. These results suggest that specific receptor sites for M. pneumoniae are markedly reduced by the induction of squamous metaplasia and, hence, appear to be specific for the normal respiratory surface containing goblet cells and pseudostratified, ciliated epithelial cells.
Full text
PDF




Images in this article
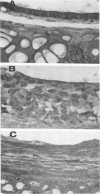
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Conrad D. H., Gockerman J. P., Gibbs M. B., Wirtz G. H. Vitamin A in liposomes. Inhibition of complement binding and alteration of membrane structure. Biochim Biophys Acta. 1975 Jun 25;394(2):157–165. doi: 10.1016/0005-2736(75)90253-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Butler M. Isolation and growth of mycoplasma in human embryo trachea cultures. Nature. 1969 Nov 8;224(5219):605–606. doi: 10.1038/224605a0. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Growth and Pathogenesis of Mycoplasma mycoides var. capri in Chicken Embryo Tracheal Organ Cultures. Infect Immun. 1970 Oct;2(4):431–438. doi: 10.1128/iai.2.4.431-438.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. E., Elin R. J. Vitamin A-induced nonspecific resistance to infection. J Infect Dis. 1974 May;129(5):597–600. doi: 10.1093/infdis/129.5.597. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Quantitative reduction of 2,3,4-triphenyl tetrazolium chloride by hamster trachea organ cultures: effects of Mycoplasma pneumoniae cells and membranes. Infect Immun. 1976 Jan;13(1):84–91. doi: 10.1128/iai.13.1.84-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Thomas L. H. Inhibition by Mycoplasma dispar of ciliary activity in tracheal organ cultures. Infect Immun. 1974 Aug;10(2):405–408. doi: 10.1128/iai.10.2.405-408.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Studies on the nature of receptors involved in attachment of tissue culture cells to mycoplasmas. Br J Exp Pathol. 1969 Feb;50(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Marchok A. C., Cone V., Nettesheim P. Induction of squamous metaplasia (vitamin A deficiency) and hypersecretory activity in tracheal organ cultures. Lab Invest. 1975 Oct;33(4):451–460. [PubMed] [Google Scholar]
- Mossman B. T., Craighead J. E. Long-term maintenance of differentiated respiratory epithelium in organ culture I. Medium composition. Proc Soc Exp Biol Med. 1975 May;149(1):227–233. doi: 10.3181/00379727-149-38778. [DOI] [PubMed] [Google Scholar]
- NEELD J. B., Jr, PEARSON W. N. Macro- and micromethods for the determination of serum vitamin A using trifluoroacetic acid. J Nutr. 1963 Apr;79:454–462. doi: 10.1093/jn/79.4.454. [DOI] [PubMed] [Google Scholar]
- Olson J. A. The biological role of vitamin A in maintaining epithelial tissues. Isr J Med Sci. 1972 Aug-Sep;8(8):1170–1178. [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge E. Mycoplasmas and cell cultures. Bacteriol Rev. 1971 Jun;35(2):206–227. doi: 10.1128/br.35.2.206-227.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Cherry J. D. A non-pathogenic mycoplasma inhibiting the effect of a pathogenic mycoplasma in organ culture. J Med Microbiol. 1972 Aug;5(3):291–298. doi: 10.1099/00222615-5-3-291. [DOI] [PubMed] [Google Scholar]
- Thomas L. Mycoplasmas as infectious agents. Annu Rev Med. 1970;21:179–186. doi: 10.1146/annurev.me.21.020170.001143. [DOI] [PubMed] [Google Scholar]
- Tvedten H. W., Whitehair C. K., Langham R. F. Influence of vitamins A and E on gnotobiotic and conventionally maintained rats exposed to Mycoplasma pulmonis. J Am Vet Med Assoc. 1973 Sep 15;163(6):605–612. [PubMed] [Google Scholar]