Abstract
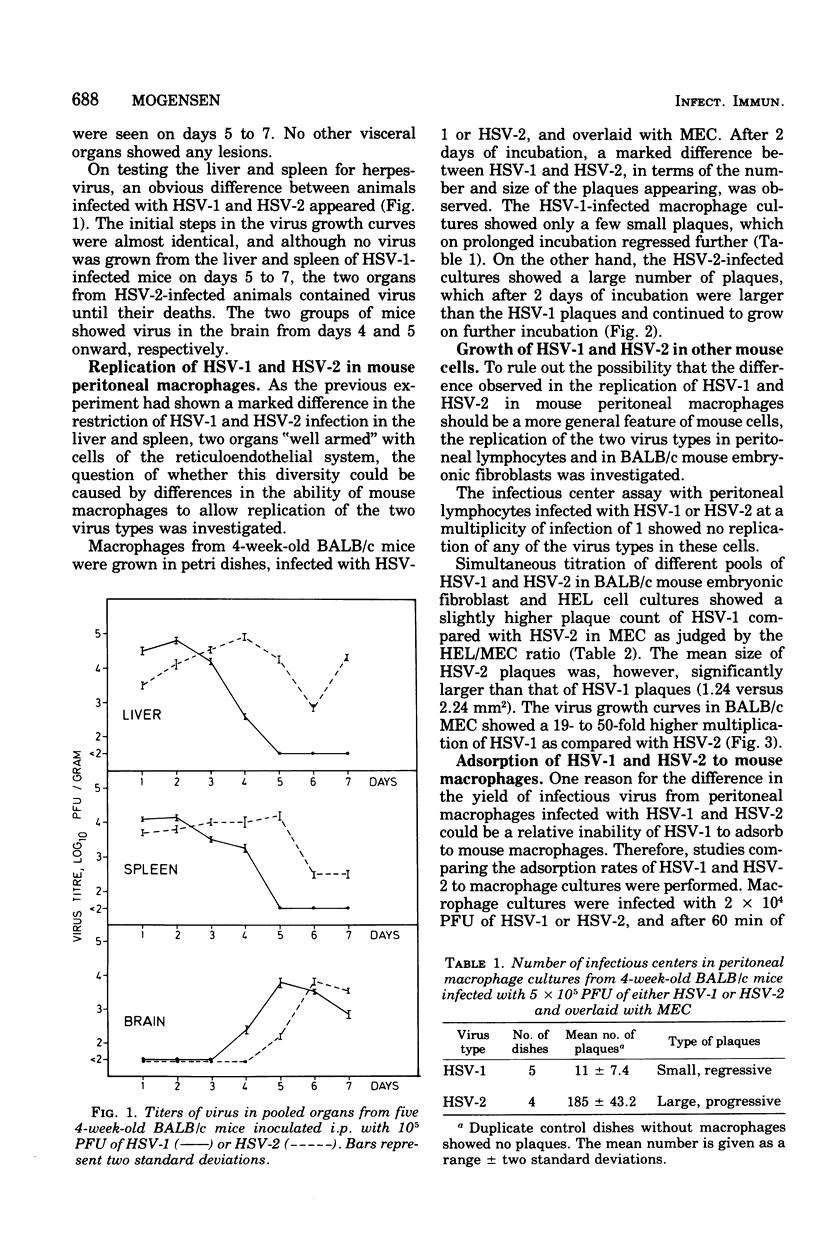
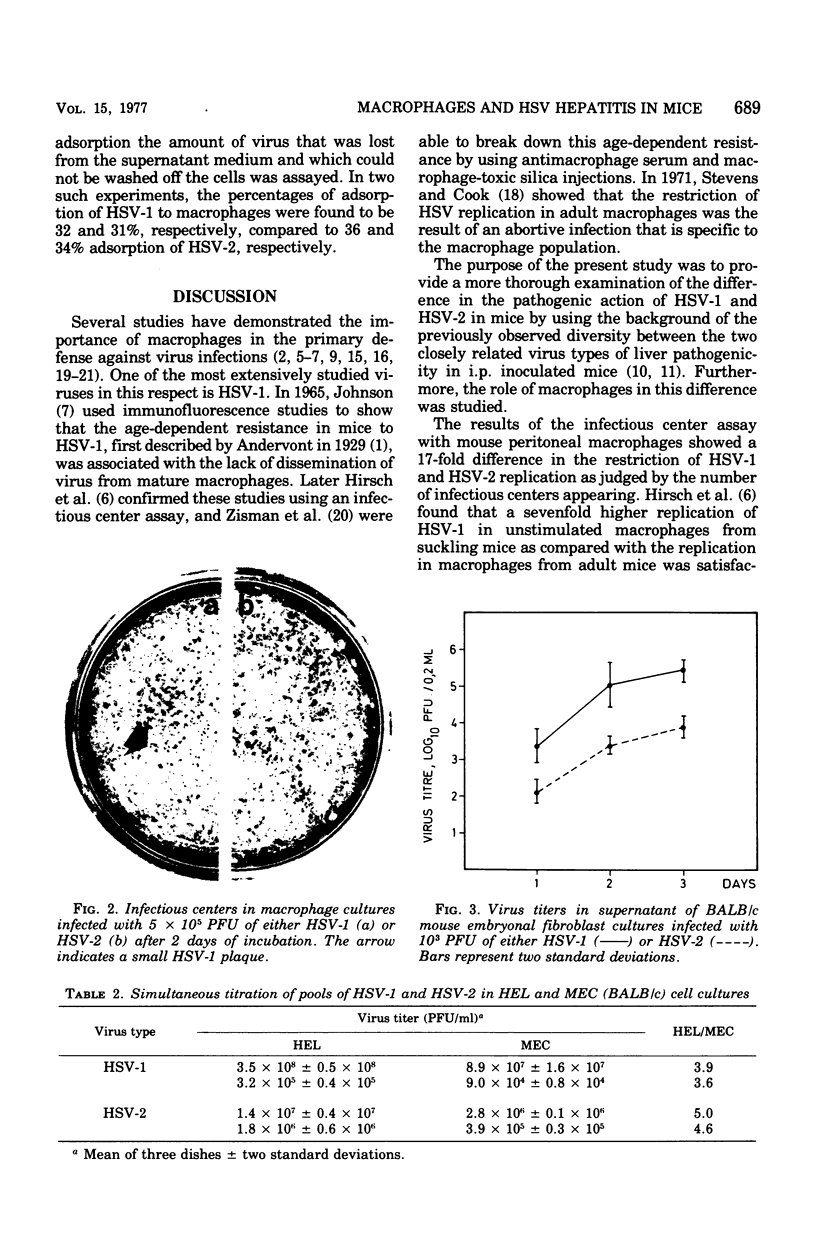
A marked difference was found between herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in the induction of hepatic necrotic lesions in mice inoculated intraperitoneally. Although HSV-2 produced many large, progressive liver lesions in 4-week-old BALB/c mice, HSV-1 only occasionally induced a few, self-limiting foci, which eventually healed. This was reflected in the isolation of HSV from the liver and spleen, two organs that are rich in macrophages. Although HSV-1 could be only temporarily isolated, HSV-2 was found in the two organs until the mice died. On the other hand, no such difference was found in the isolation of virus from the brain, which contains no macrophages, and the mice eventually died from encephalitis. This difference in hepatic involvement caused by the two virus types was found to parallel a marked difference in the restriction of HSV-1 and HSV-2 replication by macrophages as measured by an infectious center assay in vitro. HSV-2 produced 17 times as many infectious centers in infected peritoneal macrophage cultures as did HSV-1. Furthermore, the HSV-2 plaques in the cell overlay were large and increasing in size, whereas the HSV-1 plaques were small and showed regression on prolonged incubation. It was shown that this diversity was unique to the macrophage population and not caused by differences in the uptake of virus by macrophages. This model involving two closely related virus types shows the importance of tissue macrophages in the primary host defense against virus infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against Herpes simplex virus. I. Control of infection in vitro by senstized spleen cells and antibody. Infect Immun. 1973 Jun;7(6):898–904. doi: 10.1128/iai.7.6.898-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JAHMIAS A. J., KIBRICK S., ROSAN R. C. VIRAL LEUKOCYTE INTERRELATIONSHIPS. I. MULTIPLICATION OF A DNA VIRUS--HERPES SIMPLEX--IN HUMAN LEUKOCYTE CULTURES. J Immunol. 1964 Jul;93:69–74. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNK K., DONNER D. CYTOPATHISCHER EFFEKT UND PLAQUE-MORPHOLOGIE VERSCHIEDENER HERPES-SIMPLEX-VIRUSSTAEMME. Arch Gesamte Virusforsch. 1963 Aug 26;13:529–540. [PubMed] [Google Scholar]
- Mogensen S. C. Biological conditions influencing the focal necrotic hepatitis test for differentiation between herpes simplex virus types 1 and 2. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):154–158. [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Defense mechanisms against infectious bovine rhinotracheitis virus: inhibition of virus infection by murine macrophages. Infect Immun. 1975 Mar;11(3):505–511. doi: 10.1128/iai.11.3.505-511.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R. L., Centifanto Y., Kaufman H. E. Plaque size reduction as a measure of viral cell-mediated immunity. Infect Immun. 1974 Nov;10(5):1034–1039. doi: 10.1128/iai.10.5.1034-1039.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]