Abstract
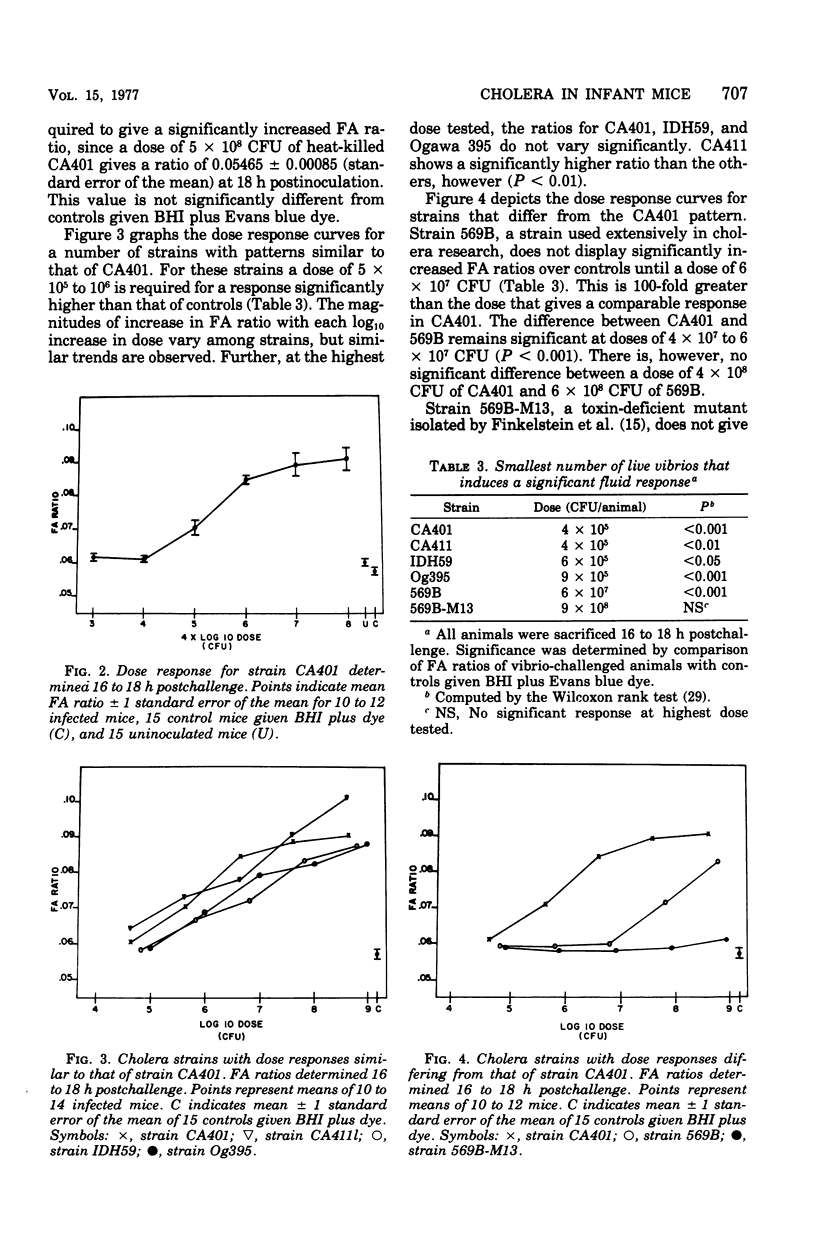
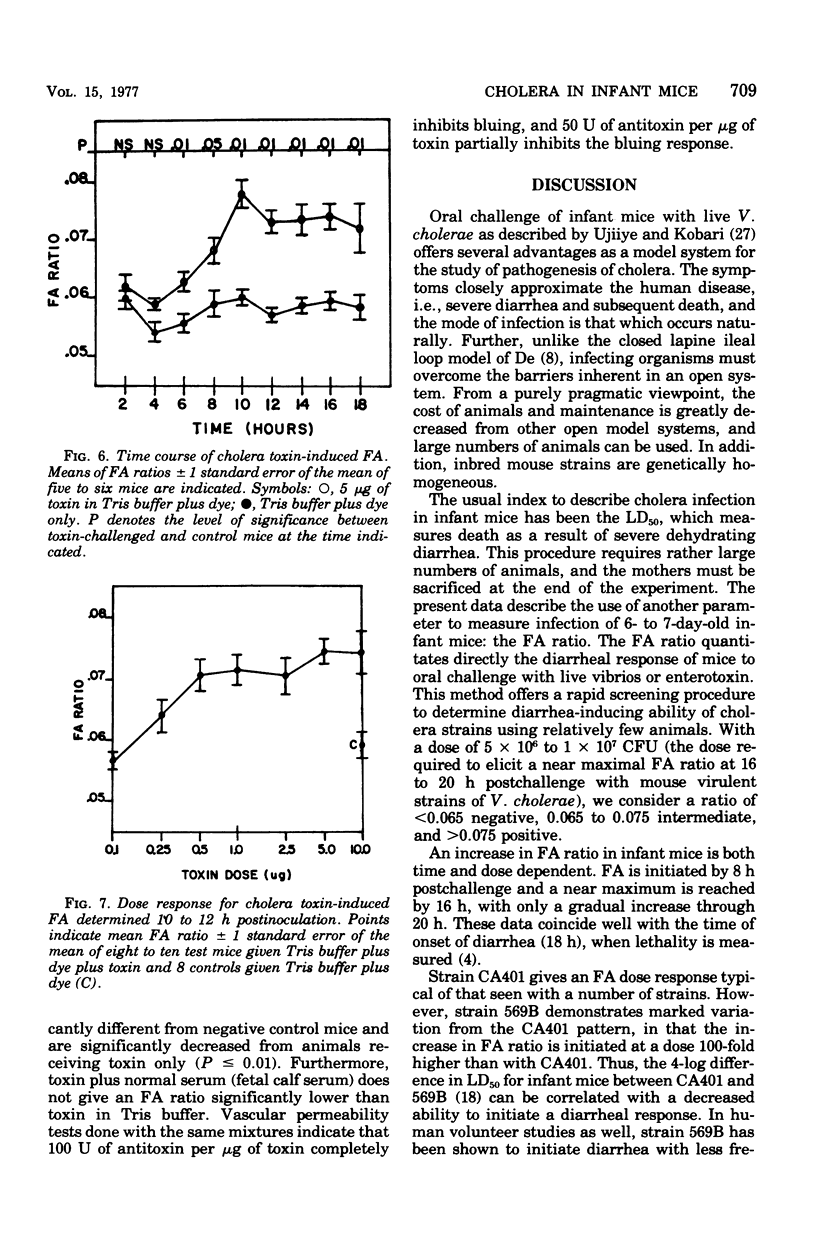
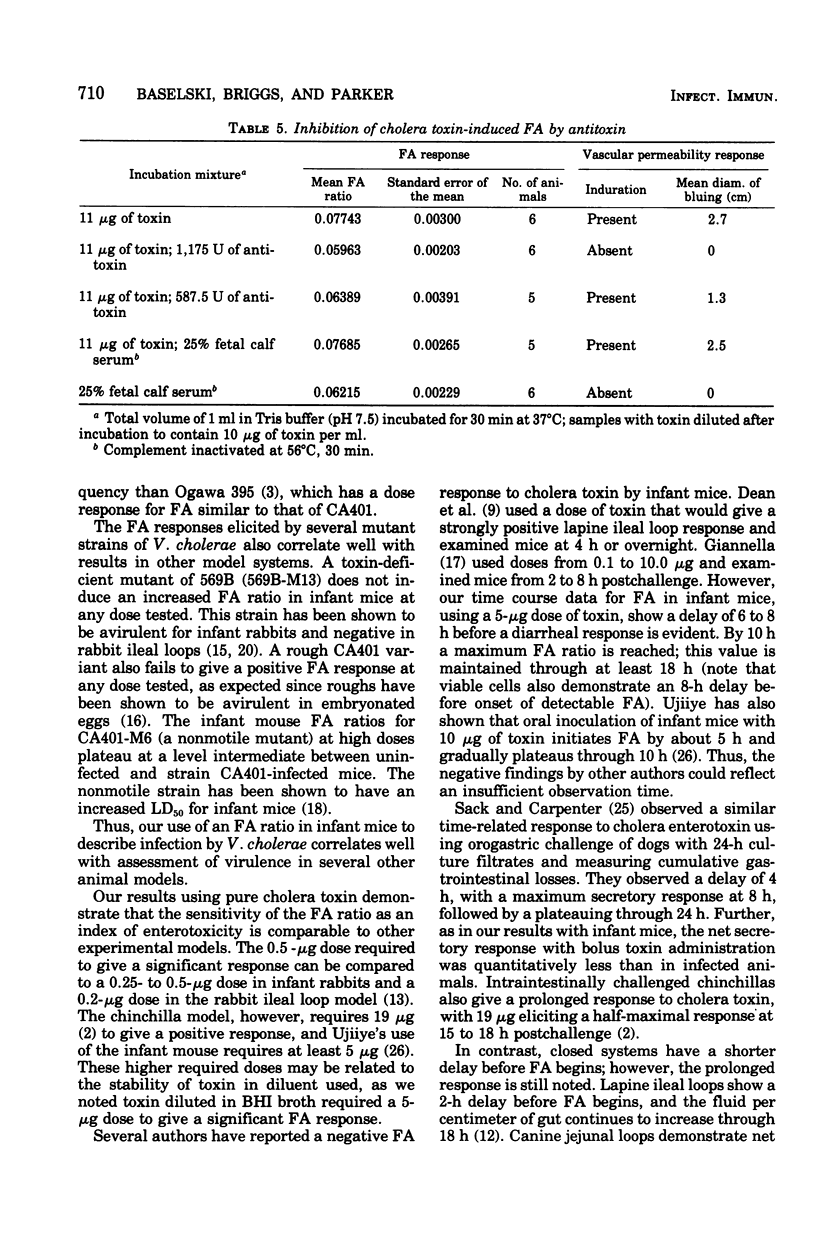
The diarrheal response of orally inoculated infant mice to viable Vibrio cholerae and purified cholera toxin was quantitated by means of a fluid accumulation (FA) ratio. The FA ratio is defined as the gut weight/remaining body weight. FA ratios were determined in relation to time of exposure and dose. Onset of fluid accumulation with viable cells of strains CA401 and 569B occurred 8 h postinoculation and reached a near maximum of 16 h. A dose of 4 x 10(6) colony-forming units of strain CA401 was required for a positive response 16 to 18 h postinoculation. Several other classical cholera strains demonstrated a similar dose-related response. Strain 569B, however, required a 100-fold higher dose to give a positive response. Several mutant cholera strains were decreased virulence in other model systems elicited FA ratios decreased from wild-type values. Onset of fluid accumulation which cholera toxin occurred 6 to 8 h postinoculation and reached a maximum by 10 h. A dose of 0.5 microng was required for a positive response 10 to 12 h postinoculation. The positive response to toxin could be inhibited by preincubation with specific antitoxin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C. Experimental cholera in humans. Br Med J. 1966 Jan 15;1(5480):140–142. doi: 10.1136/bmj.1.5480.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachman U., Goss S. J., Pickett M. J. Experimental cholera in the chinchilla. J Infect Dis. 1974 Apr;129(4):376–384. doi: 10.1093/infdis/129.4.376. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- DE S. N. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959 May 30;183(4674):1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- DUTTA N. K., HABBU M. K. Experimental cholera in infant rabbits: a method for chemotherapeutic investigation. Br J Pharmacol Chemother. 1955 Jun;10(2):153–159. doi: 10.1111/j.1476-5381.1955.tb00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTA N. K., PANSE M. V., KULKARNI D. R. Role of cholera a toxin in experimental cholera. J Bacteriol. 1959 Oct;78:594–595. doi: 10.1128/jb.78.4.594-595.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. N., Hirsh D. C. Use of the K88 antigen for in vivo bacterial competition with porcine strains of enteropathogenic Escherichia coli. Infect Immun. 1975 Jul;12(1):134–136. doi: 10.1128/iai.12.1.134-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- GARDNER E. W., LYLES S. T., LANKFORD C. E., HAGENS S. J. Vibrio cholerae infection in the embryonated egg. J Infect Dis. 1963 May-Jun;112:264–272. doi: 10.1093/infdis/112.3.264. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Chen L. C., Sharp G. W. Intestinal adenyl-cyclase activity in canine cholera: correlation with fluid accumulation. J Infect Dis. 1972 Apr;125(4):377–381. doi: 10.1093/infdis/125.4.377. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J. Biochemical properties of Escherichia coli low-molecular-weight, heat-stable enterotoxin. Infect Immun. 1974 Feb;9(2):342–347. doi: 10.1128/iai.9.2.342-347.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupal L. R., Deibel R. H. Assay, characterization, and localization of an enterotoxin produced by Salmonella. Infect Immun. 1975 Jan;11(1):14–22. doi: 10.1128/iai.11.1.14-22.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. I. Development of the model. J Infect Dis. 1969 Feb;119(2):138–149. doi: 10.1093/infdis/119.2.138. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. II. Production by cell-free culture filtrates of Vibrio cholerae. J Infect Dis. 1969 Feb;119(2):150–157. doi: 10.1093/infdis/119.2.150. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Yalow R., Berson S. A. Detection of Australia antigen and antibody by means of radioimmunoassay techniques. J Infect Dis. 1970 May;121(5):550–554. doi: 10.1093/infdis/121.5.550. [DOI] [PubMed] [Google Scholar]
- Whipp S. C., Moon H. W., Lyon N. C. Heat-stable Escherichia coli enterotoxin production in vivo. Infect Immun. 1975 Aug;12(2):240–244. doi: 10.1128/iai.12.2.240-244.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


