Abstract
Podocytes are the key cells affected in nephrotic glomerular kidney diseases, and they respond uniformly to injury with cytoskeletal rearrangement. In nephrotic diseases, such as membranous nephropathy and FSGS, persistent injury often leads to irreversible structural damage, whereas in minimal change disease, structural alterations are mostly transient. The factors leading to persistent podocyte injury are currently unknown. Proteolysis is an irreversible process and could trigger persistent podocyte injury through degradation of podocyte-specific proteins. We, therefore, analyzed the expression and functional consequence of the two most prominent proteolytic systems, the ubiquitin proteasome system (UPS) and the autophagosomal/lysosomal system, in persistent and transient podocyte injuries. We show that differential upregulation of both proteolytic systems occurs in persistent human and rodent podocyte injury. The expression of specific UPS proteins in podocytes differentiated children with minimal change disease from children with FSGS and correlated with poor clinical outcome. Degradation of the podocyte-specific protein α-actinin-4 by the UPS depended on oxidative modification in membranous nephropathy. Notably, the UPS was overwhelmed in podocytes during experimental glomerular disease, resulting in abnormal protein accumulation and compensatory upregulation of the autophagosomal/lysosomal system. Accordingly, inhibition of both proteolytic systems enhanced proteinuria in persistent nephrotic disease. This study identifies altered proteolysis as a feature of persistent podocyte injury. In the future, specific UPS proteins may serve as new biomarkers or therapeutic targets in persistent nephrotic syndrome.
Podocytes are terminally differentiated cells with a complex three-dimensional architecture embracing glomerular capillaries with their primary and interdigitating foot processes to form the glomerular sieve. Podocytes are the primary target in nephrotic syndrome, which is characterized by proteinuria, hypoalbuminemia, hyperlipidemia, and edema. Common causes of nephrotic syndrome are minimal change disease (MCD), primary FSGS, and membranous nephropathy (MGN). Patients with MGN have a variable clinical outcome. In contrast, patients with FSGS frequently present with persistent disease or patients with MCD with transient disease. Despite differences in the pathophysiology and prognosis of podocyte injury in nephrotic syndrome, the common histopathologic findings are effacement of foot processes and reduction of specific foot-process proteins in human1,2 and experimental nephrotic syndrome.3 To date, it is unknown which biochemical changes cause a reduction in foot-process protein expression.
Intracellular protein degradation is required for cellular homeostasis and is a tightly regulated process. Multiple pathways exist for the elimination of proteins. The two most prominent systems are the autophagosomal/lysosomal degradation system and the ubiquitin proteasome system (UPS). In the autophagosomal/lysosomal system, cellular organelles containing acid hydrolases (lysosomes) degrade macromolecules and organelles after phagocytosis, endocytosis, and autophagy. The UPS, however, degrades the bulk of cellular and membrane proteins. The UPS comprises enzymes, which ubiquitinate or deubiquitinate target proteins, and the 26S proteasome system, which degrades ubiquitinated proteins.4,5 In ubiquitination procedures, ubiquitin is covalently attached to target proteins by a multienzymatic system consisting of E1, E2, and E3 enzymes.6 The mode of ubiquitination determines the fate of the substrate. Whereas K48-polyubiquitination targets proteins to proteasomal degradation, K63-polyubiquitination targets proteins to autophagosomal/lysosomal degradation. Ubiquitination is a reversible process that is tightly controlled by deubiquitinating enzymes. Deubiquitinating enzymes cleave ubiquitin from proteins and disassemble polyubiquitin chains. The 26S proteasome is a multimeric protein complex that carries the proteolytic activities necessary for the degradation of polyubiquitinated proteins. The proteasome also exists in a proteolytic more effective variant called the immunoproteasome (i26S).7 Increased proteolytic activity can result in disease by removal of essential proteins required for normal cellular function.8 Impaired proteolysis can result in disease by accumulation of nondegraded material such as ubiquitinated proteins in case of UPS failure9 and storage material in lysosomes in case of lysosomal failure.10
In the kidney, altered protein degradation can lead to or influence disease.11,12 Mice with defective autophagy in podocytes develop proteinuria with age and accumulate ubiquitin.13 Ubiquitin accumulations were also described in human FSGS and human and rat MGN but not human MCD,14,15 suggesting a differential UPS status in podocytes among nephrotic diseases. Recent work showed that a prominent feature of human and rat MGN was the de novo expression of the deubiquitinating enzyme ubiquitin C-terminal hydrolase-L1 (UCH-L1) in podocytes.14,16 Inhibition of UCH-L1 reduced proteinuria and ubiquitin accumulations in rodent MGN,15 suggesting an active role of UCH-L1 and other UPS components in the persistence of podocyte disease. We, therefore, investigated whether altered proteolysis through the UPS and autophagosomal/lysosomal system occurred in nephrotic syndrome and whether proteolysis by the UPS was required for the degradation of podocyte foot-process proteins and could represent a therapeutic target.
Results
Proteolytic Systems Are Upregulated in Persistent Human Glomerular Injury
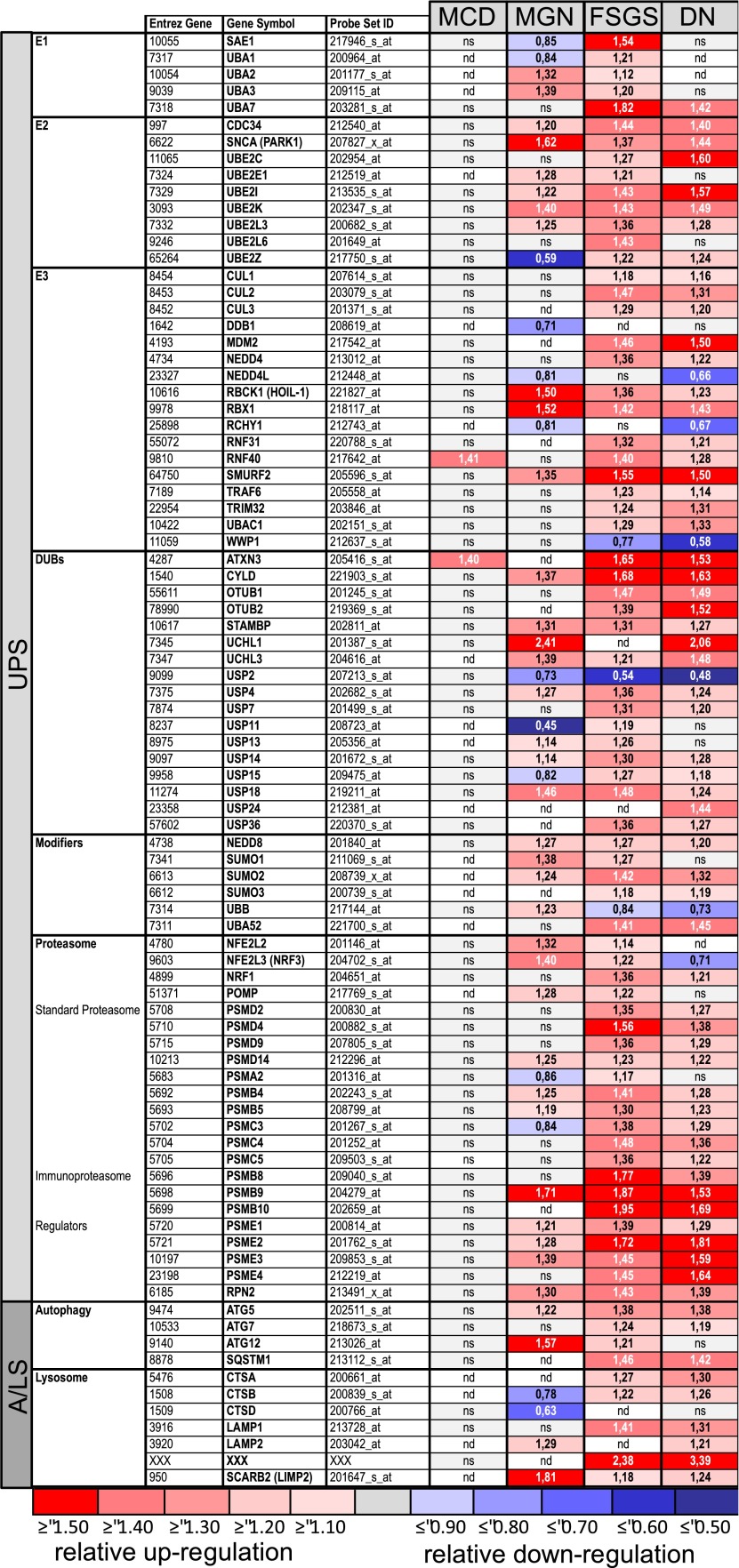
We analyzed mRNA expression levels of transcripts encoding UPS or autophagosomal/lysosomal proteins in human microdissected glomeruli. Patients with MGN, FSGS, and MCD or diabetic nephropathy (DN) as a further persistent disease were included in the analysis (Figure 1). We focused the analysis on proteolysis-related genes in the literature search. Of 128 selected genes, 87 genes were above cutoff (Figure 1), 40 genes were below cutoff (Supplemental Table 1), and 1 gene was not present on the array. In MCD, most of the evaluated transcripts were not significantly regulated. The largest number of differentially expressed genes was found in the persistent diseases FSGS and DN followed by MGN, and most of the analyzed transcripts were upregulated. Selected transcripts were specifically upregulated in MGN, such as UCH-L1 and lysosomal integral membrane protein-2 (Limp-2). In FSGS and DN, a strong upregulation of transcripts encoding for proteasome subunits and proteasome activators was noted. However, a number of transcripts were differentially expressed between MGN, FSGS, and DN. MGN and DN patients shared only 32 of 87 regulated transcripts, whereas FSGS and DN shared 68 of 87 regulated transcripts (Figure 1). Selected array data were validated by quantitative PCR (qPCR) or immunohistochemistry in biopsies from patients with MCD, MGN, and FSGS (Supplemental Figures 1 and 2). Within the glomerulus, UPS proteins were differentially expressed in podocytes, with prominent expression in MGN and/or FSGS but not in MCD.
Figure 1.
Proteolytic systems are upregulated in persistent human glomerular injury; 128 genes encoding UPS or A/LS components were selected on literature research, and their relative transcript expression levels were analyzed by microarrays in microdissected glomeruli from patients with biopsy-proven MCD (LD [n=18] versus MCD [n=5]), MGN (LD [n=14] versus MGN [n=21]), FSGS (LD [n=18] versus FSGS [n=10]), and DN (LD [n=18] versus DN [n=7]). Eighty-seven genes were above the cutoff. The cutoff was defined as (1) regulation in at least two diseases or (2) at least a 40% regulation, which means a fold change≥1.4 or ≤0.71. Heat maps of all genes with a significant regulation in MCD, MGN, FSGS, and/or DN are shown. Numbers represent fold changes compared with the respective controls (LDs). Note the difference in regulation between MCD and the more persistent glomerular injuries. DUBs, deubiquitinating enzymes; nd, not detected.
UPS Upregulation Indicated Persistent Podocyte Injury in Humans
We analyzed whether UPS upregulation in podocytes of patients indicated persistent injury. Biopsy samples of 18 children with the primary diagnosis of MCD (16 patients) and FSGS (2 patients) were analyzed for podocyte UCH-L1 expression and correlated to clinical outcome (Table 1); 14 MCD biopsies did not express UCH-L1 in podocytes, and all patients went into remission or partial remission with frequent relapses. Interestingly, two MCD biopsies showed UCH-L1 expression in podocytes, and these patients developed ESRD and received the final diagnosis of FSGS (because of the retrospective detection of a podocin mutation in one case). Both FSGS biopsies exhibited UCH-L1 expression in podocytes, and the patients developed ESRD.
Table 1.
UCH-L1 expression in podocytes suggests persistent disease
| Primary Diagnosis | UCH-L1 | Therapy | Long-Term Follow-Up | Final Diagnosis |
|---|---|---|---|---|
| MCD | 0 | Steroids | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | Steroids | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | Endoxan, CSA | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | Endoxan | Remission | MCD |
| MCD | 0 | Endoxan | Remission | MCD |
| MCD | 0 | CSA | Remission | MCD |
| MCD | 0 | Endoxan, CSA | Partial remission, frequent relapses | MCD |
| MCD | 0 | Endoxan, CSA | Frequent relapses | MCD |
| MCD | pos | Steroids, endoxan, CSA, rituiximab | ESRD, no mutation | FSGS |
| MCD | pos | CSA | ESRD, podocin mutation | FSGS |
| FSGS | pos | CSA | ESRD, podocin mutation | FSGS |
| FSGS | pos | CSA | ESRD, no mutation | FSGS |
Retrospective analysis of immunohistochemical UCH-L1 expression in podocytes of renal biopsies from patients with the primary diagnoses of minimal change disease (MCD; n=16) or FSGS (n=2). Biopsies were scored as UCH-L1–negative (0) or UCH-L1–positive (pos) independent of the number of UCH-L1–expressing podocytes. Biopsies were taken at the Department of Pediatrics between 1993 and 1998 according to standard indications for biopsy of MCD patients: (1) primary or secondary steroid resistance; (2) frequent relapses or change of treatment protocol (to Endoxan or CSA) because of steroid dependency. CSA, ciclosporin A.
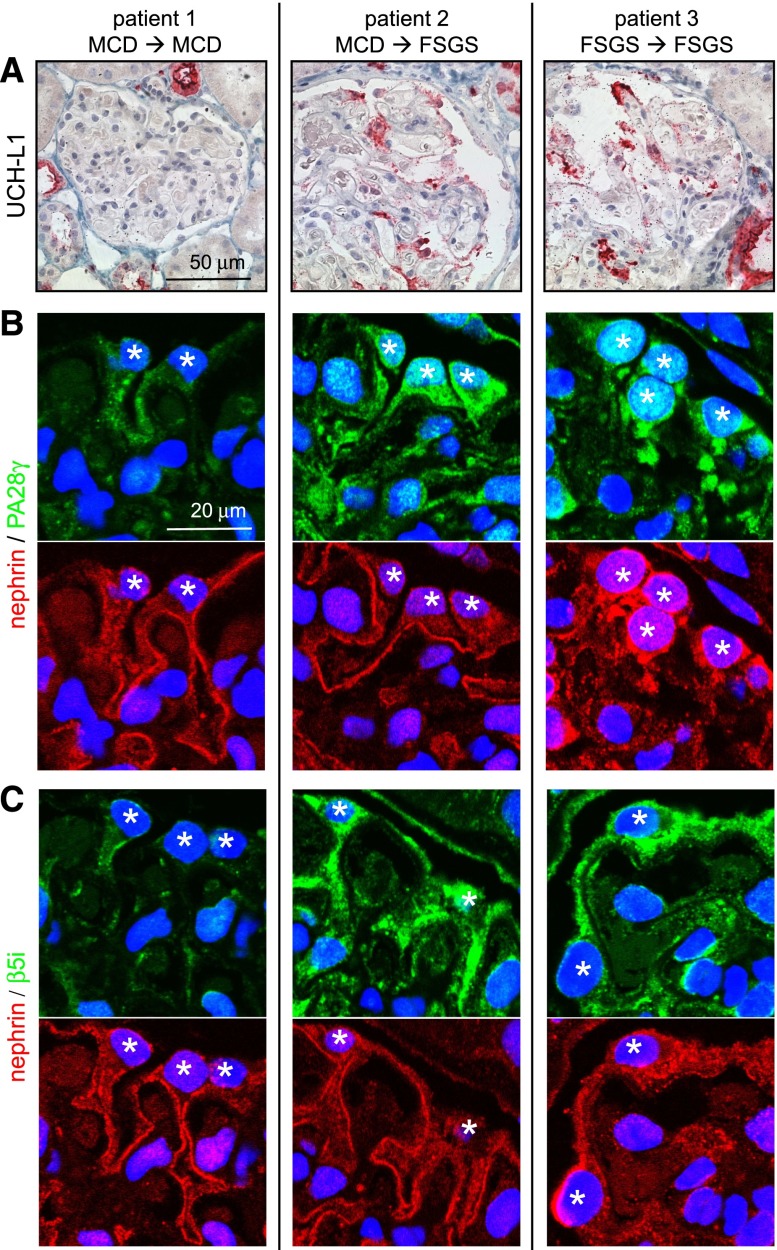
We next evaluated whether UPS markers were expressed in podocytes at the time of primary diagnosis and whether their expression correlated with development of persistent disease in these patients with MCD and FSGS (Figure 2). Proteins were chosen that suggest UPS activation, such as UCH-L1, the immuneproteasome subunit (β5i), and the proteasomal activator (PA28γ). The evaluated biopsies were dated from the time point of primary diagnosis at which patients 1 and 2 were diagnosed with MCD and patient 3 was diagnosed with FSGS. UCH-L1, β5i, and PA28γ expressions were negative in podocytes of patient 1, whereas patients 2 and 3 exhibited de novo expression of these proteins in podocytes (Figure 2). Subsequently, patient 2 progressed to ESRD and was later diagnosed as FSGS. Patient 3 also progressed to ESRD.
Figure 2.
Expression of UPS proteins in podocytes during human nephrotic syndrome is a sign for the progression to ESRD. Renal biopsies of patients with the primary diagnosis of MCD (patients 1 and 2) or FSGS (patient 3) were stained for UPS proteins. (A) Deubiquitinating enzyme UCH-L1 (red). (B) Proteasome activator PA28γ (green; costained with nephrin [red]). (C) β5i unit of the immunoproteasome (green; costained with nephrin [red]). Patients 2 and 3 received the end diagnosis FSGS during clinical follow-up (→) and progressed to ESRD. Patient 1 went into remission and maintained the diagnosis of MCD. Note the increased expression of UPS proteins in patient 2 in podocytes with intact nephrin staining. *Podocyte nucleus.
Proteolytic Systems Are Upregulated in Persistent Rat Glomerular Injury
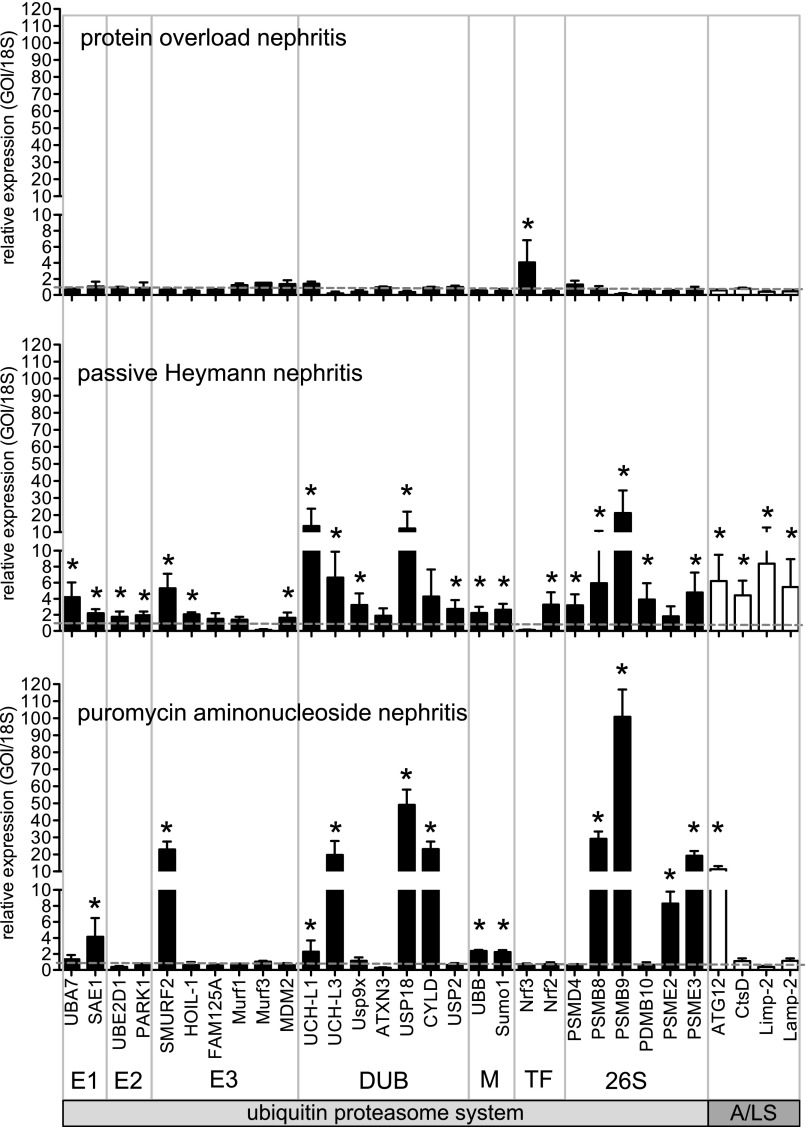
Increased podocyte expression of UPS and autophagosomal/lysosomal proteins discriminated patients with MGN and FSGS from patients with MCD. We, therefore, investigated whether glomerular upregulation of these proteolytic systems also discriminated persistent from transient podocyte injury in rodents (Figure 3). Transient proteinuria was induced in the protein overload model (POL). Persistent podocyte injury was induced in the models of passive Heymann nephritis (PHN) and puromycin aminonucleosid nephritis (PAN) as detailed in Concise Methods. In POL, most UPS and autophagosomal/lysosomal transcripts were not significantly regulated by qPCR, despite strong proteinuria. The only significant upregulation was noted for the transcription factor Nrf3, which represses the gene transcription of proteasome subunits.17 Persistent injury in PHN or PAN rats, however, induced UPS protein transcripts, such as proteasome subunits and proteasomal activators. PAN induced the strongest changes in UPS components. In PHN but not PAN, the transcription factor Nrf2, which induces the gene transcription of proteasome subunits,18 was significantly induced as well as proteins required for normal autophagosomal/lysosomal function. The observed changes in expression profiles in rodents resembled those changes observed in glomeruli of patients with MCD, MGN, FSGS, and DN.
Figure 3.
Proteolytic systems are differentially upregulated in persistent rat glomerular injury. Relative glomerular expression of transcripts encoding for ubiquitin proteasome players (black bars: E1, ubiquitin activating enzymes; E2, ubiquitin binding enzymes; E3, ubiquitin ligating enzymes; DUB, deubiquitinating enzymes; M, modifiers [ubiquitin or ubiquitin-like proteins]; TF, transcription factors; 26S, proteasome subunits) or players involved in the autophagosomal/lysosomal degradation system in rats (A/LS; white bars). Transient podocyte injury was induced by BSA injection (protein overload; n=4; day 2). Persistent podocyte injury was induced by injection of FX1A serum (PHN; n=6; 18 days) or PAN (n=4; 4 weeks) as described in detail in Concise Methods. Values were normalized to 18S RNA as the housekeeping gene of the same preparations, and the relative expressions to controls were calculated. *P<0.05 was accepted as statistically significant to respective controls (dashed gray line=1). GOI, gene of interest.
Foot-Process Proteins Are Decreased in Persistent Human and Rat Glomerular Injury
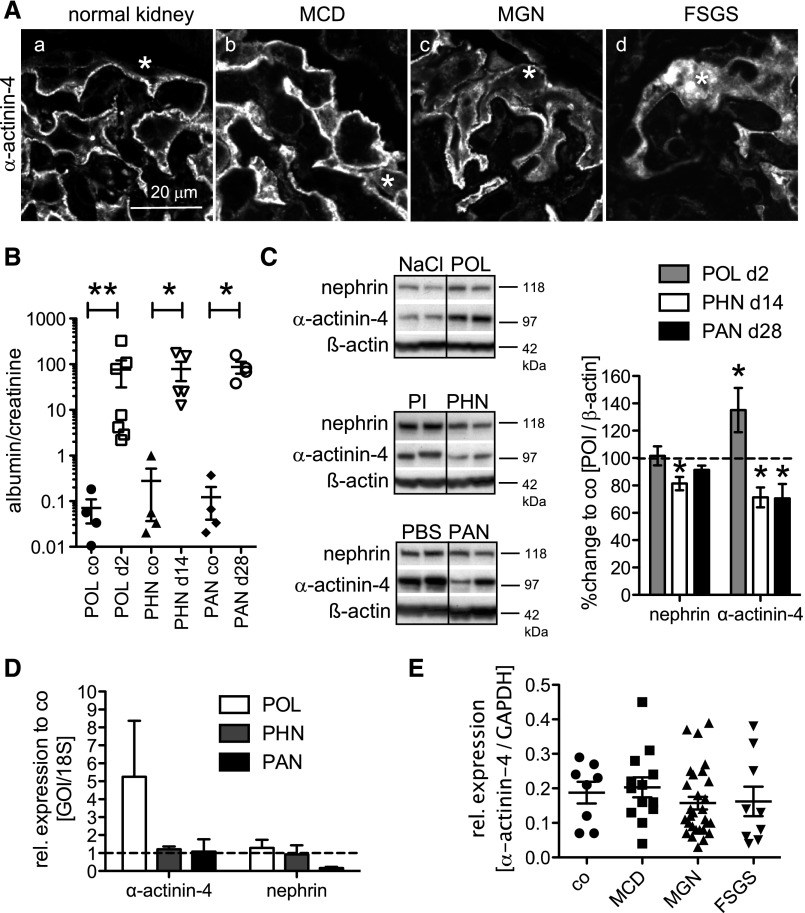
Upregulation of proteolytic systems could cause persistent disease by degradation of functional podocyte proteins. Immunofluorescent analysis showed a disrupted α-actinin-4 staining in biopsies from patients with MGN and FSGS but not MCD (Figure 4A). We, therefore, analyzed whether glomerular protein levels of α-actinin-4 and nephrin were reduced in rats with persistent (PHN and PAN) but not transient (POL) podocyte disease at a time point of comparable proteinuria (Figure 4B). In POL, glomerular α-actinin-4 content was increased, and nephrin content was not changed (Figure 4C). The increased α-actinin-4 content coincided with increased transcript levels (Figure 4D). In PHN, nephrin and α-actinin-4 contents were reduced; in PAN, only glomerular α-actinin-4 content was reduced (Figure 4C). The decrease of nephrin and α-actinin-4 protein content was not a consequence of reduced transcript levels in rat and human glomeruli (Figure 4, D and E), suggesting a proteolytic rather than a transcriptional downregulation of both proteins in persistent podocyte injury.
Figure 4.
Foot-process proteins, such as nephrin and α-actinin-4, are decreased in persistent human and rat glomerular injury. (A) Confocal micrographs of α-actinin-4 immunolocalization in renal biopsy specimens of control (a), MCD (b), MGN (c), or FSGS (d). *Podocyte cell body. Note disrupted α-actinin-4 staining in MGN and FSGS. (B–D) Transient podocyte damage was induced in rats through intraperitoneal BSA injection day 1, and persistent podocyte damage was induced through intravenous injection of FX1A serum day −1 and day 0 or intraperitoneal injection of puromycin amino nucleoside. (B) Urine albumin-to-creatinine ratio at the day of euthanization (day 2, POL control [NaCl] and POL; day 18, PHN control [PI] and PHN; week 4, PAN control [PBS] and PAN). *P<0.05; **P<0.01. (C, left) Western blotting for nephrin and α-actinin-4 from isolated glomeruli of POL rats on day 2, PHN rats on day 18, and PAN rats on day 28 after disease induction. (C, right) Densitometric analysis of nephrin and α-actinin-4 levels from two independent experiments with n=3–4 samples per condition. Values were normalized to β-actin of the same membrane and are expressed as percent change to control rats (co; dashed line). *P<0.05. POI, protein of interest. (D) Relative glomerular mRNA expression levels of nephrin and α-actinin-4 in POL glomeruli (n=6; day 2), PHN glomeruli (n=6; 14 days), or PAN glomeruli (n=6; 4 weeks) to respective controls. Values were normalized to 18S RNA as a housekeeping gene of the same preparations and are expressed as mean±SEM; no statistical significance was noted to respective controls (dashed gray line=1). (E) Relative expression levels of α-actinin-4 mRNA in microdissected human glomeruli of LD (co; n=8) or biopsy-proven MCD (n=14), MGN (n=29), and FSGS (n=9). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal housekeeper to adjust for unequal total mRNA content. GOI, gene of interest.
The UPS Degrades Foot-Process Proteins in Persistent Podocyte Injury
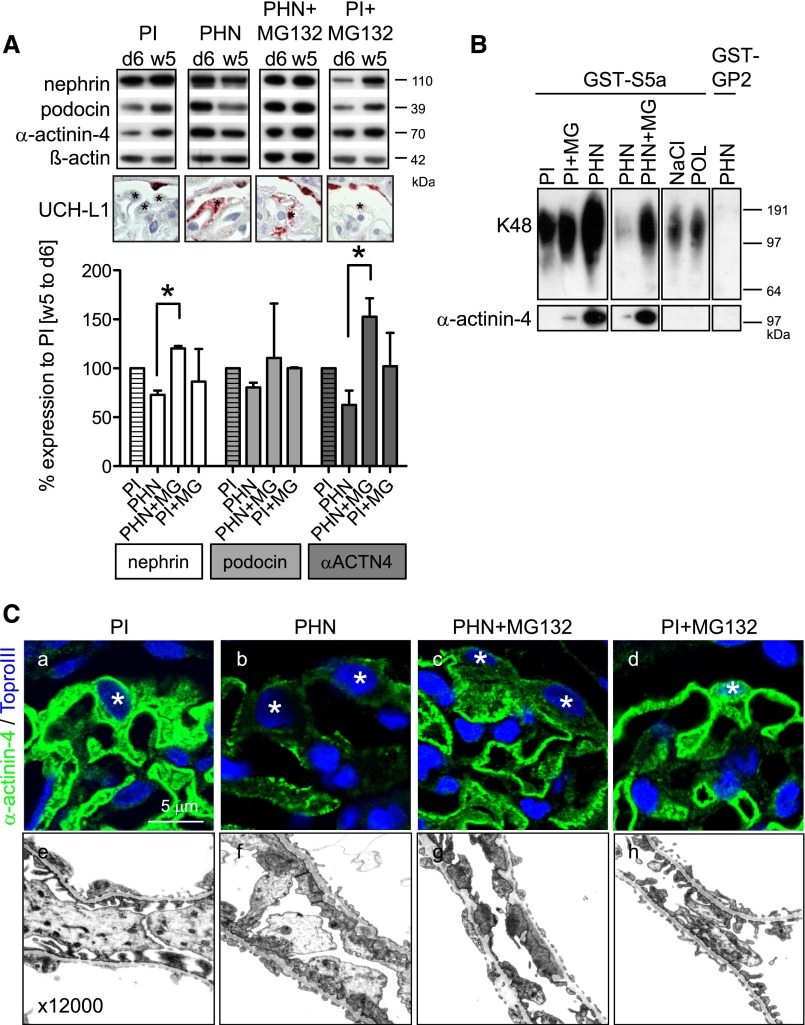
To allow a sequential analysis of foot-process protein content in one individual rat, rats were uninephrectomized on day 6 after injection of PHN or preimmune serum (PI). Uninephrectomy converts PHN from a model with stable renal function to a progressive model.19 Levels of nephrin, podocin, and α-actinin-4 were reduced in PHN glomeruli at week 5 compared with day 6, and UCH-L1 was expressed in podocytes as a surrogate marker for UPS upregulation. Three days before removal of the second kidney in week 5, proteasomal degradation was inhibited by treatment with MG132. Inhibition of proteasomal degradation led to stabilization of nephrin, podocin, and α-actinin-4 contents in PHN glomeruli (Figure 5A).
Figure 5.
The UPS degrades foot-process proteins in persistent podocyte injury. (A) Inhibition of proteasomal degradation stabilizes nephrin, podocin, and α-actinin-4 in PHN rats. PHN was induced in rats day −1 and day 0 through intravenous injection of FX1A (PHN) or PI (control). On day 6, a unilateral nephrectomy was performed, and glomeruli were isolated. Proteasomal activity was inhibited with MG132 (60 μg/kg body wt) for 3 days before euthanization at week 5. (Top) Western blotting for nephrin, podocin, and α-actinin-4 from day 6- and week 5-isolated glomeruli from the same rat shows reduction of foot-process proteins in PHN rats, which is abolished by inhibition of the proteasome. β-Actin of the same membrane was used as a loading control. (Middle) Micrographs of UCH-L1 expression (red) as a surrogate marker of UPS upregulation in respective rats at week 5. *Podocytes. (Bottom) Densitometric analysis of percent changes of nephrin, podocin, and α-actinin-4 levels from week 5 to day 6 glomeruli of individual rats (n=2–3) compared with control rats (PI; striped bars). *P<0.05. (B) In a different set of experiments, K48-polyubiquitinated proteins were pulled down from glomerular lysates through the GST-S5a matrix. PHN was performed without uninephrectomy to provide sufficient glomerular protein per rat 28 days after disease induction with or without prior proteasomal inhibition for 2 weeks. Upper panel shows increased pull down of K48-polyubiquitinated proteins from 100 μg glomerular lysate in PHN rats and PHN rats with proteasomal inhibition by Western blotting for ubiquitin. After induction of transient podocyte injury in the model of protein overload day 2 (POL), no increased content of K48-polyubiquitinated proteins was noted to NaCl control-injected rats. GST-GP2 matrix was used to control for unspecific pull down (lane 8, long exposition). The same membrane was then subjected to Western blotting for K48-polyubiquitinated α-actinin-4 within the precipitate. Note the increased content of K48-polyubiquitinated α-actinin-4 in PHN rats (lane 3), which was further increased by proteasomal inhibition (lane 5). No K48-polyubiquitinated α-actinin-4 was detected in POL rats. Three independent experiments were performed. (C) Immunolocalization (a–d) of α-actinin-4 and electron micrographs (e–h) in PI and PHN rats 5 weeks after disease induction after a 3-day course of proteasomal inhibition. *Podocyte nucleus.
Next, we analyzed whether foot-process proteins were within the fraction of K48-polyubiquitinated proteins (Figure 5B). K48-polyubiquitination targets proteins to proteasomal degradation through binding to the proteasomal S5a subunit. Pull-down experiments of K48-polyubiquitinated proteins with glutathione transferase (GST)–tagged S5a from glomerular lysates of PHN and POL rats showed K48-polyubiquitinated α-actinin-4 in PHN but not POL or control. Furthermore, inhibition of proteasomal degradation with MG132 for 2 weeks starting 14 days after disease induction led to additional stabilization of K48-polyubiquitinated α-actinin-4 in PHN glomeruli, showing a functional role of the UPS in the degradation of α-actinin-4 in persistent injury. However, proteasomal inhibition led to prominent granular α-actinin-4 staining in the cytoplasm and at the filtration barrier, and foot-process effacement was unchanged after proteasomal inhibition in PHN rats (Figure 5C). In addition, proteinuria was increased but not significantly when proteasomal inhibition commenced early (day 4 for 14 days) or late (week 5 for 3 days) in PHN (Supplemental Figure 3). Of note, late and long proteasomal inhibition (day 14 for 14 days) increased proteinuria in PHN rats significantly.15
The UPS in Human Podocytes and Rat Persistent Podocyte Injury Degrades Oxidized α-Actinin-4
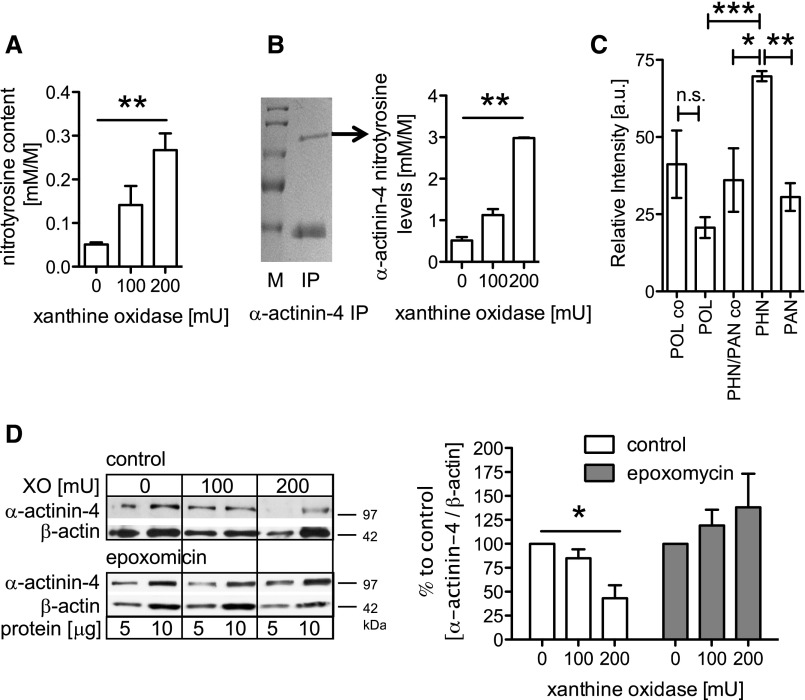
Because rescue of glomerular α-actinin-4 levels by proteasomal inhibition did not ameliorate persistent disease, we hypothesized that damaged α-actinin-4 was stabilized. Commonly, the UPS degrades oxidative damaged proteins. To test for reactive oxygen species (ROS)–mediated modification of α-actinin-4, free radicals were generated in cultured human podocytes using the xanthine (X) and xanthineoxidase (XO) system.20 A dose-dependent increase of 3-nitrotyrosine content was observed in podocyte lysates (Figure 6A) and immunoprecipitated α-actinin-4 by mass spectrometry (Figure 6B) of human podocytes exposed to X/XO. ROS-mediated α-actinin-4 modification was also found in isolated glomeruli of POL, PAN, and PHN rats. Three α-actinin-4 peptides containing a dioxidized tryptophan were identified (Supplemental Table 2), and the amount was highest in PHN compared with PAN glomeruli (Figure 6C, Supplemental Figure 4B) and unchanged in POL glomeruli. Because α-actinin-4 was a target of oxidative damage in vivo and in vitro, we evaluated whether α-actinin-4 was degraded by the proteasome in response to ROS. Indeed, a dose-dependent loss of α-actinin-4 protein was observed in cultured human podocytes exposed to X/XO, which was prevented by pretreatment with the proteasome-specific inhibitor epoxomicin (Figure 6D).
Figure 6.
The UPS in human podocytes and rat persistent podocyte injury degrades damaged α-actinin-4. (A) Human podocytes were treated for 5 hours with different concentrations of XO and analyzed for total nitrotyrosine content as a marker for oxidative stress (n=3). (B) α-Actinin-4 protein was immunopurified from untreated and XO-treated podocytes and separated using SDS-PAGE followed by a coomassie staining (left; arrow, α-actinin-4). IP, immunoprecipitate; M, marker. (Right) The α-actinin-4 protein band was cut from the gel and analyzed for nitrotyrosine modifications using MS. A significant increase of nitrotyrosine modifications within the α-actinin-4 protein was seen in samples exposed to XO (n=2). (C) Immunopurified α-actinin-4 from glomerular lysates of PHN day 14 (n=2), PAN week 4 (n=3), and POL day 2 (n=2) treated rats and respective control rats (POL co, n=2; PHN/PAN co, n=3) was separated by SDS-PAGE and subjected to MS analysis for oxidative modifications. The amounts of the identified tryptic α-actinin-4 peptide M*TLGM*IW**TIILR (m/z=756.2) [M+2H]2+ with a dioxidized tryptophan (W**) and two oxidized methionines (M*) are shown relative to an unmodified α-actinin-4 peptide. (D, left) Western blotting for α-actinin-4 from protein lysates of human podocytes exposed to 5 hours of XO with or without pretreatment with the proteasome inhibitor epoxomicin (10 μM). Two concentrations of total protein lysates (5 and 10 μg) were loaded for reference. (D, right) Densitometric analysis of three to four independent experiments with n=1 sample per condition. Values were normalized to β-actin of the same membrane and are expressed as percent change to control (0 mU XO). *P<0.5; **P<0.01; ***P<0.001.
Proteasomal Proteolytic Capacity Is Overwhelmed before the Development of Proteinuria in Persistent Podocyte Injury
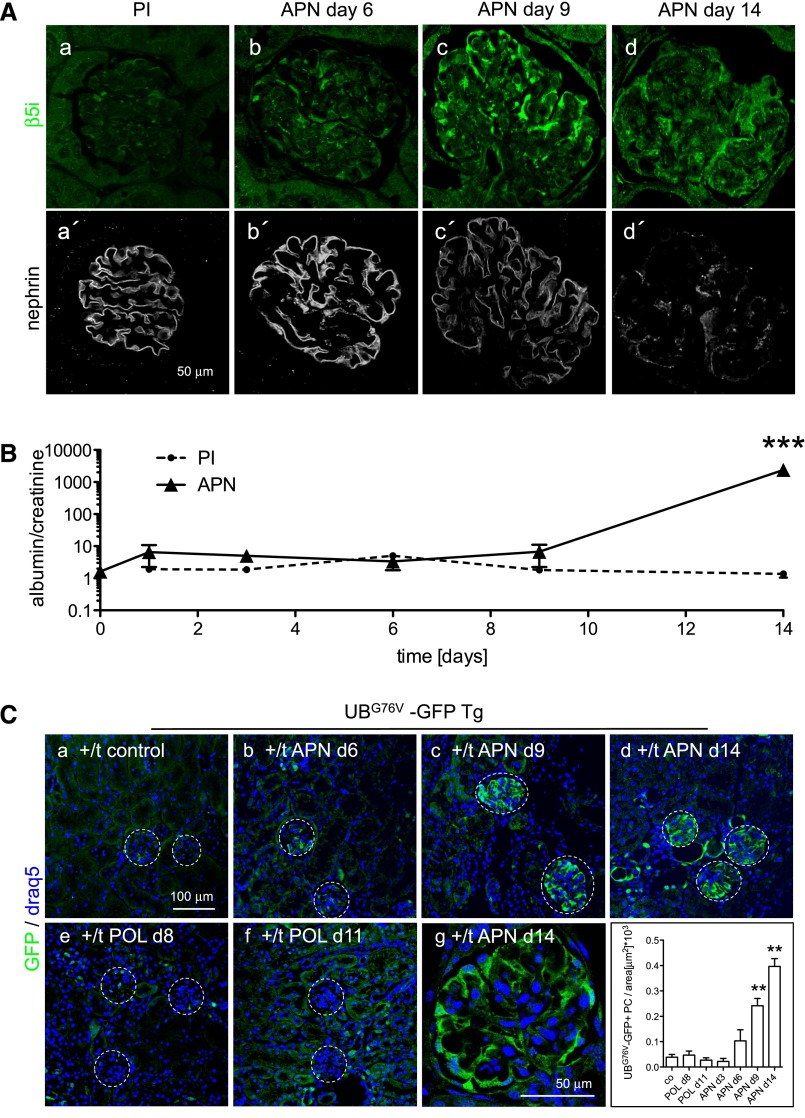
An upregulation of UPS proteins in podocytes does not necessarily indicate effective proteasomal removal of targeted proteins. Ineffective proteolysis could also trigger persistent disease.21 We, therefore, assessed the proteasomal capacity by using UBG76V-green fluorescent protein (GFP) transgenic mice. These mice express GFP tagged to a constitutively active proteasomal degradation signal in the form of mutant UBG76V. Sufficient proteolytic capacity leads to immediate proteasomal degradation of UBG76V-GFP, whereas impairment of the proteasome results in accumulation of UBG76V-GFP.22 The model of antipodocyte nephritis (APN)23 was used to induce persistent podocyte injury. APN results in subepithelial immune complex deposition and nephrotic syndrome 9–14 days after disease induction.24 We investigated whether UPS upregulation (visualized by β5i expression) and UPS capacity (UBG76V-GFP accumulation) correlated with foot-process protein disruption and the development of proteinuria (Figure 7). In APN, β5i expression was mostly restricted to podocytes and observed on day 3 focally and days 6–14 globally in podocytes (Figure 7A, a–d). Disruption of nephrin staining and the development of proteinuria lagged behind the increased β5i expression in podocytes of APN mice (Figure 7, A, a′–d′, and B). Induction of APN resulted in UBG76V-GFP accumulation specifically in podocytes and before the development of proteinuria and disruption of nephrin staining, with maximal UBG76V-GFP accumulations on day 14 (Figure 7C, c, d, and g). In contrast, induction of transient podocyte injury through protein overload did not result in UBG76V-GFP accumulation in podocytes during maximal proteinuria on day 8 or after recovery to control levels by day 11 (Figure 7C, e and f, Supplemental Figure 5B). These experiments showed proteasomal impairment in podocytes in persistent but not transient proteinuric injury.
Figure 7.
Proteasomal proteolytic capacity is overwhelmed before the development of proteinuria in persistent podocyte injury. (A) Confocal images of staining for the immunoproteasome subunit β5i (a–d; green) and nephrin (a′–d′; white) days 6, 9, and 14 after induction of APN or in a PI-injected control mouse day 14 show an early expression of β5i in podocytes of APN mice before disrupted nephrin staining. (B) Time course of development of albuminuria measured by ELISA. Values were normalized against urine creatinine of respective urine samples. ***P<0.001. (C) Confocal images of staining for GFP (green) in UBG76V-GFP transgenic mice 14 days after injection of PI or APN serum show early accumulation of UBG76V-GFP in podocytes of APN mice. Induction of reversible podocyte injury in the model of POL did not result in UBG76V-GFP accumulation in podocytes during (day 8; e) and after (day 11; f) intraperitoneal administration of BSA for 7 subsequent days. (g) Glomerular close-up view of an UBG76V-GFP transgenic mouse day 14 after APN induction. (h) GFP-positive podocytes were counted in 20 glomeruli and normalized against glomerular area in the different mouse groups. **P<0.001
The Autophagosomal/Lysosomal System Compensates for Impaired UPS Function
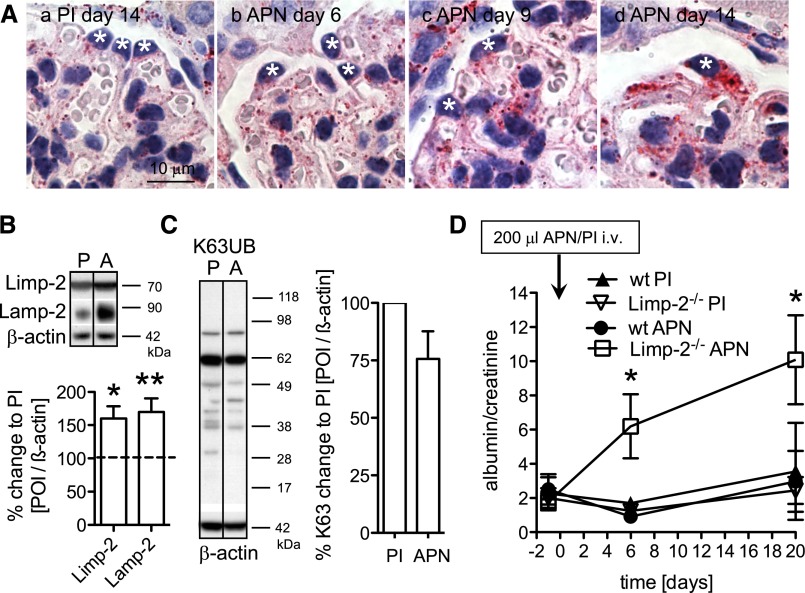
Impairment of the UPS can be compensated for by the autophagosomal/lysosomal system,25 and upregulation of the autophagosomal/lysosomal system was detected in persistent human and rodent podocyte injury (Figures 1 and 3). We therefore assessed autophagosomal/lysosomal protein expression and function in APN (Figure 8). Contrasting the early UPS upregulation, the number of Limp-2–positive lysosomes was predominantly elevated on day 14 in podocytes along with glomerular Limp-2 and lysosomal-associated membrane protein-2 levels in APN mice (Figure 8, A, d, and B). Autophagosomal/lysosomal upregulation, therefore, occurred when the UPS was globally impaired in podocytes. Overwhelmed lysosomal degradation is characterized by accumulation of K63-polyubiquitinated proteins. However, levels of K63-polyubiquitinated proteins were slightly decreased in APN glomeruli on day 14 (Figure 8C), suggesting intact removal by lysosomes. To test the functional consequence of the autophagosomal/lysosomal system upregulation, we induced APN in Limp-2–deficient mice. Proteinuria developed earlier and stronger in APN-treated Limp-2–deficient mice compared with control littermates, showing that lysosomal proteolysis attenuated the course of APN (Figure 8D).
Figure 8.
The autophagosomal/lysosomal system compensates for impaired UPS function. APN was induced day 0 by intravenous injection of 300 μl sheep APN or unspecific sheep PI (control). (A) Staining for the lysosomal protein Limp-2 in APN mice and a PI-injected control mouse shows a significant accumulation of lysosomes in podocytes on day 14 in APN. (B) Lysosomal Limp-2 and lysososmal-associated membrane protein-2 (LAMP-2) levels are increased in isolated glomeruli of APN mice (A) relative to PI (P=1.00; dotted line) day 14 mice treated by Western blotting and densitometric analysis. Values were normalized to β-actin of the same membrane and are expressed as percent change to PI mice (n=3–6 animals per time point and condition). (C) K63-polyubiquitinated proteins from glomerular lysates of PI and APN mice were quantified day 14 by Western blotting and densitometric analysis (n=3 mice per condition). (D) Time course of urine albumin-to-creatinine ratio in Limp-2–deficient mice and control littermates after induction of mild APN through intravenous (i.v.) injection of 200 μl APN or PI serum (n=3–4 for all conditions and time points). *P<0.05; **P<0.01.
Discussion
In this study, upregulation of proteolytic systems specifically occurred in patients with MGN, FSGS, and DN and was a sign of persistent but not transient human and rodent podocyte injury. The data suggest that UPS upregulation may be an early marker of FSGS in children with nephrotic syndrome, which differentiates FSGS from MCD. Both diseases exhibit similar pathologic findings of normal light microscopy and generalized foot-process effacement in early stages. Segmental glomerular scarring occurs only in later stages of FSGS and thereafter, distinguishing FSGS from MCD. Histologic screening of more patients is required to determine if UPS upregulation can serve as a prognostic indicator of persistent disease. Additional studies will also have to address the different primary and secondary disease entities that lead to FSGS or MGN and study the proteolytic setup in podocytes in correlation to disease activity and progression. Of note, the transcriptome pattern from isolated glomeruli may not be specific for persistent podocyte injury, because concordant expression changes were also noted in DN, with additional endothelial and mesangial cell injury.26 Accordingly, the analysis of regulated transcripts of MGN, FSGS, and DN revealed differential regulation of many transcripts, indicating specific proteasomal and autosomal/lysosomal pathway upregulation depending on the underlying pathology. Interestingly, the upregulation pattern of proteolysis also differed within the rodent podocyte injuries. In PHN, levels of both UPS and autophagosomal/lysosomal transcripts were increased, whereas in PAN, predominantly UPS transcripts were elevated.
In MGN and FSGS, disease persistence is characterized by continuous injury by autoantibodies27 or soluble factors.28 In rodent29–32 and human MGN,33 reactive oxygen species are strong stressors and known to induce the UPS.34–37 In functional studies, we show that UPS activity reduced foot-process proteins in PHN. Specifically, α-actinin-4 was degraded by the UPS after oxidative modification in PHN. Future studies may explore the role of phospholipase A2 receptor antibody deposition in primary MGN in the induction of oxidative modification of podocyte proteins and upregulation of the UPS. Modulation of such oxidative modification may also represent a future target of therapy in human MGN.
In persistent podocyte injury, the proteolytic capacity by the UPS was increased through (1) upregulation of the immunoproteasome, which enables a more effective removal of proteins,7 (2) an increase of proteasomal activators, which enhances the entry of K48-polyubiquitinated proteins into the proteasome, and (3) a de novo expression of deubiquitinating enzymes (such as UCH-L1), which increases the available pool of free ubiquitin required for ubiquitination. Are these alterations in the UPS even a possible cause of persistent disease? UPS upregulation occurred before disruption of foot processes and proteinuria in APN and therefore, could trigger progression by removal of important regulatory proteins. Inhibition of proteasomal degradation could then exert beneficial effects in rodent MGN, which has been shown in animal models of stroke38 and necrotizing crescentic GN.39 However, despite reducing foot-process protein degradation, broad proteasomal inhibition increased proteinuria in PHN in our study, arguing for proteolysis by the UPS as a reparative rather than a causative mechanism of disease. A similar observation was obtained concerning the autophagosomal/lysosomal system. Mice with defective autophagosomal/lysosomal degradation caused by Limp-2 or autophagy related 5 deficiency13 exhibited an accelerated course of glomerular disease. Upregulation of the autophagosomal/lysosomal system can occur to compensate for UPS impairment,25 similar to our findings with autophagosomal/lysosomal system upregulation in the setting of UPS impairment and proteinuria. Podocytes accumulate polyubiquitinated protein aggregates in human and rodent MGN,15 which are typical substrates for autophagosomal/lysosomal degradation.40 Importantly, autophagosomal/lysosomal system impairment, which can also occur in case of severe oxidative stress,41 did not occur.
Other than UPS activation, UPS impairment could trigger persistent disease in podocytes (as described in the nervous system21) through accumulation of nondegraded proteins, such as oxidative damaged α-actinin-4, which might cause cytotoxic effects.42 We found that podocytes were prone to UPS impairment in persistent but not transient podocyte injury. Therefore, restoration of proteasomal function by modulation of specific UPS proteins,15 by enhancement of proteasomal activity,43,44 or through specific activation of proteases45 may be an appropriate therapeutic strategy in persistent podocyte injury. Future studies will aid in determining whether UPS activation is associated with persistent podocyte injury, and additional work is required to determine if UPS and autophagosomal/lysosomal system upregulation is the consequence of such persistent injury or contributes to it. For the first time, this study identifies altered proteolysis as a prominent feature of persistent podocyte injury. In the future, specific UPS proteins may serve as new biomarkers or therapeutic targets in persistent nephrotic syndrome.
Concise Methods
Antibodies
Primary antibodies used for the study were mouse α-UCH-L1 (1:40, TSA amplification, clone 13C4; Abcam, Inc.), guinea pig α-nephrin (Acris), rabbit α-α-actinin-4 (Immunoglobe), goat α-podocin (Santa Cruz Biotechnology), rabbit α-PA28γ (Novus Biologicals), rabbit α-β5i (Abcam, Inc.), rabbit α-ubiquitin (Chemicon), rabbit α-K63 (Apu3; EMD Millipore), mouse α-β-actin (Sigma-Aldrich), rabbit α-Limp-2 (Paul Saftig), rabbit α-lysosomal–associated membrane protein-2 (Sigma-Aldrich), and rabbit α-GFP (Abcam, Inc.). All secondary antibodies used were either biotinylated or fluorescent dye-conjugated affinity-purified donkey antibodies (Jackson ImmunoResearch Laboratories). All plastic ware was from Falcon or Sarstedt.
Animal Experiments
POL was induced in 200- to 250-g Wistar rats by intraperitoneal injection of BSA in NaCl (Sigma-Aldrich) as described.46 Intraperitoneal injection of high doses of BSA results in increased transcapillary albumin transport, leading to degenerative changes in podocytes with reversible development of large pore defects.47 Animals were euthanized on day 2 for additional analyses when proteinuria was highest. PHN, which is widely accepted as a model for human MGN,48,49 was induced in 200- to 250-g male Sprague–Dawley rats by intravenous injection of 500 μl sheep anti-FX1A antiserum or control sheep preimmune serum followed by 750 μl sheep anti-FX1A antiserum or control sheep preimmune serum the next day. Anti-FX1A antiserum was induced by repeated immunizations of sheep with isolated brush borders of proximal tubuli from rat kidneys as described.50 Injection of heterologous antisera to rat renal tubular brush border antigen extract leads to proteinuria from sublytic podocyte injury induced by the complement membrane attack complex of C5b-951 and through subepithelial immune deposits. PAN was induced in Sprague–Dawley rats by intraperitoneal injection of puromycin aminonucleoside in PBS (Sigma-Aldrich) as described.52 Puromycin aminonucleoside induces severe proteinuria and glomerular ultrastructural changes in rats by direct injury of the podocyte cytoskeleton,53 leading to sustained foot-process effacement, podocyte loss, glomerular sclerosis, and proteinuria resembling human FSGS. Animals were euthanized on day 14, 18, 28, 35 (PHN), or 28 (PAN) for additional analyses when persistent injury with morphologic changes, such as focal sclerosis in PAN and podocyte swelling and loss in PHN, was established. For proteasomal inhibition, three treatment regiments were used. (1) Rats were uninephrectomized day 6 after injection of PHN or day 0 of PI; 4 weeks later, PHN and PI rats were treated with 60 μl/kg body wt MG132 over subcutaneous osmotic minipumps (Alzet) for 3 days before removal of the second kidney on week 5 to evaluate whether foot-process protein levels could be increased on proteasomal inhibition. PHN rats were treated (2) 4 or (3) 14 days after disease induction with 60 μl/kg body wt MG132 over subcutaneous osmotic minipumps (Alzet) to investigate whether preventive or therapeutic proteasomal inhibition ameliorated clinical course of disease. APN was induced in C57BL/6, Limp-2–deficient, and UBG76V-GFP transgenic mice by intravenous injection of 200–300 μl antipodocyte serum or PI as described.23 For time course experiments, kidneys were harvested on days 3, 6, 9, and 14. Protein overload was induced in UBG76V-GFP transgenic mice though daily intraperitoneal injections of increasing doses of BSA (4, 6, 8, 10, 10, 10, and 10 mg/ml) in PBS for 7 days as described.13 Rats and mice were housed in a controlled animal facility with free access to water and standard animal chow. The animal experiments were performed according to national and institutional animal care and ethical guidelines and have been approved by the local ethic committees.
Cell Culture and Treatments
The conditionally immortalized human podocyte cell line was a gift from M.A. Saleem (Bristol, UK). Cell culture was performed as already described.54 Human podocytes were exposed to 150 µM xanthine (Sigma-Aldrich) and 50–200 mU xanthine-oxidase (Sigma-Aldrich) to induce oxidative stress. To block proteasome activity within the podocytes, 5 or 10 µM epoxomicin (Calbiochem) was used. Control cells were treated in parallel with the solvent of epoxomixin (DMSO).
Human Specimen Collection
The study was conducted according to the Declaration of Helsinki principles with approval from the local ethics committee. Tissue samples were obtained from the Nierenregister of the University Medical Center Hamburg-Eppendorf. For the retrospective analysis, biopsy samples of 18 patients with the primary diagnosis of MCD or FSGS were collected in the Department of Pediatric Nephrology between 1988 and 2006 according to standard indications for biopsy of MCD patients: (1) primary or secondary steroid resistance and (2) frequent relapses or change of treatment protocol because of steroid dependency. Clinical data of these patients were collected between 1988 and 2010.
Immunohistochemistry
One-micrometer paraffin sections were deparaffinized, and antigen retrieval was performed by microwave boiling (10 mM citrate buffer [pH 6.1]) or protease digestion (protease XXIV, 5 mg/ml; Sigma-Aldrich). Unspecific binding was blocked (5% horse serum; 30 minutes at room temperature); 1° antibody incubations (5% horse serum over night [o/n], 4°C) were followed by incubation with biotinylated or AF488- or Cy3-coupled 2° antibodies (1:400, 30 minutes at room temperature). Color development was performed with the TSA kit (NEN) according to the manufacturer’s instructions with neufuchsin, and nuclei were counterstained with hematoxylin. Stainings were evaluated under an Axioskop and photographed with an Axiocam HRc using the Axiostar software or an LSM 510 β-microscope using the LSM software (Carl Zeiss).
Patients and Microarray Analyses
Human renal biopsy specimens and Affymetrix microarray expression data were procured within the framework of the European Renal cDNA Bank—Kröner–Fresenius Biopsy Bank.55 Diagnostic renal biopsies were obtained from patients after informed consent with approval of the local ethics committees. After renal biopsy, the tissue was transferred to RNase inhibitor and microdissected into glomerular and tubular fragments. Total RNA was isolated from microdissected glomeruli, reverse-transcribed, and linearly amplified according to a protocol previously reported.56 The microarray expression data used in this study came from individual patients with MCD, MGN, FSGS, and DN. Pretransplantation kidney biopsies from living donors (LDs) were used as control renal tissue. Fragmentation, hybridization, staining, and imaging were performed according to the Affymetrix Expression Analysis Technical Manual (Affymetrix). For microarray analysis, robust multichip analysis was performed. After normalized robust multichip analysis, significance analysis of microarrays was conducted using a q value of less than 5% to identify genes that were differently regulated between the analyzed groups.57 The significance analysis of microarrays q value can be interpreted as analogous to a P value that is corrected for multiple testing; 128 genes encoding ubiquitin proteasome or autophagosomal/lysosomal system components were selected on literature research, and their relative transcript expression levels were analyzed by Affymetrix microarrays in microdissected glomeruli from patients with biopsy-proven MCD (HG-U133Plus 2.0 Array: LD [n=18] versus MCD [n=5]), MGN (HG-U133A array: LD [n=14] versus MGN [n=21]), FSGS (HG-U133Plus 2.0 Array: LD [n=18] versus FSGS [n=10]), and DN (DN7; HG-U133Plus 2.0 array: LD [n=18] versus DN [n=7]). Probe sets, which were present on both array types and significantly regulated in at least one disease, were considered for additional evaluation. Because some of the genes on the array were represented by several probe sets, we selected those probe sets that were significantly regulated in more than one disease. If still more than one probe set fulfilled this criterion, we looked for the probe set with the highest fold change. To further filter for genes, which might help differentiate between transient and persistent podocyte injury, we defined another cutoff. Only genes/probe sets that showed either regulation in at least two diseases or at least 40% regulation, meaning a fold change ≥1.4 or ≤0.71, were included (Figure 1).
Patients qPCR Analyses
Reverse transcription and real-time RT-PCR were performed as reported earlier.58 Predeveloped TaqMan reagents were used for human UCH-L1 (NM_004181.3) as well as the reference genes, 18S rRNA, and glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems). The expression of the candidate gene was normalized to the reference genes. The mRNA expression was analyzed by standard curve quantification.
Rat qPCR Analyses
Total messenger RNA was extracted from isolated glomeruli with the RLT Plus Kit (Qiagen) according to the manufacturer’s instructions, and 1 μg was reverse-transcribed with random hexamer primer (Invitrogen) and MMLV reverse transcription (New England Biolabs). mRNA expression was quantified with an AbiPrism NN8860 using SYBR green as recently described.59 The exon spanning primer pairs used are listed in Supplemental Table 3; 18S was used as an internal control to correct for small variations in RNA quality and cDNA synthesis essentially as described by AbiPrism. Amplicons of random samples for each primer pair were determined by automatic PCR sequencing to show the specificity of the PCR reaction (data not shown). Relative quantification of gene expression was calculated using the ΔΔCT method.
Immunoblot
Immunoblots were performed as described previously60 from isolated glomeruli. Briefly, samples were lysed in T-PER (Pierce; containing 1 mM sodium fluoride, 1 mM sodium vanadate, 100 nM calyculin A, complete [Roche], and 2 μM epoxomicin; Calbiochem) and denatured with 4× lithium dodecyl sulfate. Samples were separated on a 4%–12% Bis⋅Tris NuPage gel (Invitrogen) in NuPage running buffer. Protein transfer was performed in transfer buffer (50 mM Tris base and 0.192 M glycine in ddH2O) in a Novex Mini Cell (Invitrogen). Polyvinylidenefluoride membranes (EMD Millipore) were blocked (3% nonfat milk) before incubation with 1° antibodies diluted in Superblock blocking reagent (Pierce). Binding was detected by incubation with horseradish peroxidase–coupled 2° antibodies (1:10,000; 3% nonfat milk). Protein expression was visualized with ECL SuperSignal (Pierce) according to the manufacturer’s instructions on a Biomax Light Film (Kodak). Western blots were analyzed using software from ImageJ.61
GST-S5a Pull Down
GST-S5a pull down of K48-linked polyubiquitinated proteins was performed as described.62 In brief, 100 μg total glomerular protein lysate (T-PER; Pierce; 1 mM sodium fluoride, 1 mM sodium vanadate, 100 nM calyculin A, complete [Roche], and 2 μM epoxomicin) was incubated with 20 μl (10 μg) GST-S5a or for control, GST-GP2–coupled glutathione-sepharose (GE Healthcare) beads o/n at 4°C. After centrifugation, pellets were washed five times with Tris-buffered saline /Tween 20 for 5 minutes at 4°C. Pellets were resuspended in 20 μl SDS loading buffer (1% SDS, 50 mM dithiothreitol, 50 mM Tris⋅HCl [pH 6.8], 0.005% bromphenol-blue, and 10% glycerol), denatured for 5 minutes at 95°C, and resolved for Western blot against K48-polyubiquitin and α-actinin-4 as a reprobe.
Immunoprecipitation
Whole-cell extracts were adjusted to a concentration of 500 µg in 500 µl 1× lysis buffer, 1% Triton X-100, 75 mM NaCl, 50 mM Hepes (pH 7.4), 1 mM EGTA, 1 mM Na2VO4, and complete protease inhibitor cocktail (Roche). A preclear step was performed using 30 µl ProteinG sepharose beads (Sigma-Aldrich). Samples were centrifuged for 10 minutes at 14,000 rpm, and supernatants were transferred into a new tube o/n at 4°C with 7.5 µg α-actinin-4 antibody. Negative controls were performed with 7.5 µg unspecific rabbit-IgG (Sigma-Aldrich). Subsequently, 30 µl ProteinG sepharose was added and incubated for 3 hours at 4°C to immobilize the immune complex. After 10 minutes of centrifugation at 14,000 rpm, the supernatant was discarded, and the pellets were washed three times with 1 ml 1× lysis buffer each and dissolved in 4× loading buffer for SDS-PAGE.
Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Analyses in Multiple Reaction Monitoring Mode of Total Cell Lysates and Immunopurified Samples
Amino acid hydrolysis was performed on total cell lysates and digested α-actinin-4 protein; 30 μg cell lysate protein diluted in 1 ml 50 mM sodium phosphate buffer (pH 7.4) was precipitated with ice-cold trichloroacetic acid (10% v/v) collected by centrifugation (3000 rpm for 10 minutes at 4°C) and delipidated one time with water/methanol/water saturated diethyl ether (1:3:7, v/v/v).63 Immunoprecipitated α-actinin-4 was resolved using SDS-PAGE, excised, and digested with trypsin (Protein In-Gel Digestion Kit; Agilent Technologies). To the respective samples, 500 µl 4N methanesulfonic acid (Sigma-Aldrich) pretreated with 10 mg/ml benzoic acid was added. Oxidative modified tyrosines were quantified by liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) with multiple reaction monitoring mode in the positive ion acquisition mode using an Agilent 6410 triple quadrupole MS system equipped with an Agilent 1200 LC system after the addition of isotopically labeled internal standards as described previously.64
HPLC-ESI–Ion Trap–MS/MS Analyses of Immunoprecipitated Glomerular α-Actinin-4
Immunoprecipitated α-actinin-4 was resolved by SDS-PAGE. For tryptic in-gel digestion, gel bands corresponding to α-actinin-4 were cut into small pieces (1×1 mm) and transferred into a 1.5-ml tube. Gel samples were destained by alternating washes with 100 mM ammonium bicarbonate (NH4HCO3, swelling buffer; Sigma-Aldrich) and 100% acetonitrile (ACN; Merck). Disulfide bonds were reduced with DTT (10 mM in 100 mM NH4HCO3; Sigma-Aldrich) for 10 minutes at 56°C. Cysteins were alkylated by iodacetamide (55 mM dissolved in 100 mM NH4HCO3; Sigma-Aldrich) for 30 minutes in the dark. Protein digestion was carried out at 37°C for 16 hours with trypsin (sequencing grade; Promega) dissolved in digestion buffer (50 mM NH4HCO3, 10% ACN/HPLC-grade water; Merck) to a final concentration of 13 ng/µl. After tryptic digestion, the supernatant was transferred from the gel pieces into a new collection tube. Peptides were extracted by 65% ACN/5% formic acid (v/v) in HPLC-grade water. The supernatant was transferred into the collection tube, and a second extraction was carried out with ACN. The extracted peptide solution in the collection tube was then evaporated to complete dryness using a vacuum centrifuge (4–16 K; Christ) equipped with a cooling trap (RCF10; Thermo Fisher Scientific). Before mass spectrometric protein identification, the samples were dissolved in 1 µl 50% ACN and 9 µl 0.2% formic acid (v/v). Mass spectrometric protein identification by LC-ESI–ion trap–MS (ion trap, XCT; Agilent Technologies) equipped with an HPLC-chip system (Chip Cube; Agilent Technologies) was already described elsewhere.65 Deviating details are briefly described here. The HPLC-chip contained polystyrene/divinylbenzene reversed phase analytic column (300 Å, 5 μM). For LC-MS analysis, 8 μl tryptically digested α-actinin-4 samples were injected. The elution gradient consisted of 3%–30% solvent B within 30 minutes. The generic files for database searching were generated from LC-ESI–ion trap–MS data by the software data analysis for 6300 Series Ion Trap LC-MS, Version 4.0 (Agilent Technologies). Protein identification was performed by Mascot software (Version 2.4.01) using the UniProtKB database (http://www.uniprot.org/; specifically searching in the rat protein database [rattus]). Search parameters included one potential trypsin cleavage site (missed cleavage), a mass tolerance of ±1.2 Da for the precursor ions and ±0.6 Da for the fragment ions, and the variable protein modifications carbamidomethyl and oxidation. Additionally, variable protein modifications were included for dioxidized W, P, R, K, F, Y, and C. Three tryptic α-actinin-4 peptides with dioxidized W were identified in all samples, with individual ions scores>41 indicating identity or extensive homology (P<0.05). Peptide sequences, mascot scores, and peptide retention times are denoted in Supplemental Table 2. For relative quantification of the dioxidized peptides, extracted ion chromatograms were generated. The peak areas (area under the curves) of the corresponding peaks in the extracted ion chromatograms were determined and normalized against the area under the curves of an unmodified α-actinin-4 peptide (ETTDTDTADQVIASFK), which was present in all samples and did not show any additional modified forms.
Statistical Analyses
Values are means±SEMs; n refers to the number of animals or individual measurements in separate samples. Two groups were compared using the unpaired t test for the comparison of mean values with equal variance, whereas the Mann–Whitney U test was used for the comparison of mean values with unequal variance of different experimental series. Comparison of three or more groups was performed by a one-way ANOVA/Tukey post-test for parametric data or the Kruskall–Wallis/Dunns post-test for nonparametric data. A P value <0.05 was accepted as statistically significant and calculated using Microsoft Excel or GraphPad Prism 5 software.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Ursula Kneissler and the technical assistant trainees Alena Osthaar-Ebker, Marie Mohr, and Jennifer Boldt for excellent help; Michael Schwake and Paul Saftig for the Limp-2–deficient mice and the Limp-2 antibody; XueJun Wang for the β5 antibody; Saskia Schlossarek, Lucie Carrier, and Nico Dantuma for the UBG76V-GFP transgenic mice; and Moin Saleem for the immortalized human podocytes. We thank all participating centers of the European Renal cDNA Bank—Kroener–Fresenius Biopsy Bank (ERCB-KFB) and their patients for their cooperation. Active members at the time of the study are listed in ref. 56.
M.B., V.R., and J.-H.K. were supported by the Integrated Research Training Group of CRC877 (Christian Albrechts University, Kiel, Germany). This study was supported by Deutsche Forschungsgesellschaft Grant KFO288 (to C.M.-S.) and, in part, National Institute of Diabetes and Digestive and Kidney Diseases O’Brien Kidney Center at the University of Michigan Grant P30-DK081943.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050522/-/DCSupplemental.
References
- 1.Guan N, Ding J, Zhang J, Yang J: Expression of nephrin, podocin, alpha-actinin, and WT1 in children with nephrotic syndrome. Pediatr Nephrol 18: 1122–1127, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Huh W, Kim DJ, Kim MK, Kim YG, Oh HY, Ruotsalainen V, Tryggvason K: Expression of nephrin in acquired human glomerular disease. Nephrol Dial Transplant 17: 478–484, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Luimula P, Sandström N, Novikov D, Holthöfer H: Podocyte-associated molecules in puromycin aminonucleoside nephrosis of the rat. Lab Invest 82: 713–718, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Ciechanover A, Orian A, Schwartz AL: The ubiquitin-mediated proteolytic pathway: Mode of action and clinical implications. J Cell Biochem Suppl 34: 40–51, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Hochstrasser M: Biochemistry. All in the ubiquitin family. Science 289: 563–564, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Pickart CM: Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schröter F, Prozorovski T, Lange N, Steffen J, Rieger M, Kuckelkorn U, Aktas O, Kloetzel PM, Krüger E: Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142: 613–624, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Rajan V, Mitch WE: Ubiquitin, proteasomes and proteolytic mechanisms activated by kidney disease. Biochim Biophys Acta 1782: 795–799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardley HC, Scott GB, Rose SA, Tan NG, Robinson PA: UCH-L1 aggresome formation in response to proteasome impairment indicates a role in inclusion formation in Parkinson’s disease. J Neurochem 90: 379–391, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Berkovic SF, Dibbens LM, Oshlack A, Silver JD, Katerelos M, Vears DF, Lüllmann-Rauch R, Blanz J, Zhang KW, Stankovich J, Kalnins RM, Dowling JP, Andermann E, Andermann F, Faldini E, D’Hooge R, Vadlamudi L, Macdonell RA, Hodgson BL, Bayly MA, Savige J, Mulley JC, Smyth GK, Power DA, Saftig P, Bahlo M: Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet 82: 673–684, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lott JS, Coddington-Lawson SJ, Teesdale-Spittle PH, McDonald FJ: A single WW domain is the predominant mediator of the interaction between the human ubiquitin-protein ligase Nedd4 and the human epithelial sodium channel. Biochem J 361: 481–488, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC: Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer-Schwesinger C, Meyer TN, Münster S, Klug P, Saleem M, Helmchen U, Stahl RA: A new role for the neuronal ubiquitin C-terminal hydrolase-L1 (UCH-L1) in podocyte process formation and podocyte injury in human glomerulopathies. J Pathol 217: 452–464, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Schwesinger C, Meyer TN, Sievert H, Hoxha E, Sachs M, Klupp EM, Münster S, Balabanov S, Carrier L, Helmchen U, Thaiss F, Stahl RA: Ubiquitin C-terminal hydrolase-l1 activity induces polyubiquitin accumulation in podocytes and increases proteinuria in rat membranous nephropathy. Am J Pathol 178: 2044–2057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Wu J, Wu H, Wang T, Gan H, Zhang X, Liu Y, Li R, Zhao Z, Chen Q, Guo M, Zhang Z: UCH-L1 expression of podocytes in diseased glomeruli and in vitro. J Pathol 217: 642–653, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Sankaranarayanan K, Jaiswal AK: Nrf3 negatively regulates antioxidant-response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. J Biol Chem 279: 50810–50817, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW: Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23: 8786–8794, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler S, Striker LJ, Striker GE, Perkinson DT, Hibbert J, Couser WG: Studies of progressive glomerular sclerosis in the rat. Am J Pathol 123: 553–562, 1986 [PMC free article] [PubMed] [Google Scholar]
- 20.Cutaia M, Parks N: Effect of hyperoxia and exogenous oxidant stress on pulmonary artery endothelial cell Na+/H+ antiport activity. J Lab Clin Med 128: 154–164, 1996 [DOI] [PubMed] [Google Scholar]
- 21.McNaught KS, Mytilineou C, Jnobaptiste R, Yabut J, Shashidharan P, Jennert P, Olanow CW: Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem 81: 301–306, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lindsten K, Menéndez-Benito V, Masucci MG, Dantuma NP: A transgenic mouse model of the ubiquitin/proteasome system. Nat Biotechnol 21: 897–902, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Schwesinger C, Dehde S, Klug P, Becker JU, Mathey S, Arefi K, Balabanov S, Venz S, Endlich KH, Pekna M, Gessner JE, Thaiss F, Meyer TN: Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J Immunol 187: 3218–3229, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Borza DB, Zhang JJ, Beck LH, Jr., Meyer-Schwesinger C, Luo W: Mouse models of membranous nephropathy: The road less travelled by. Am J Clin Exp Immunol 2: 135–145, 2013 [PMC free article] [PubMed] [Google Scholar]
- 25.Korolchuk VI, Menzies FM, Rubinsztein DC: Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett 584: 1393–1398, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wehner H, Petri M: Glomerular alterations in experimental diabetes of the rat. Pathol Res Pract 176: 145–157, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Beck LH, Jr.: Monoclonal anti-PLA2R and recurrent membranous nephropathy: Another piece of the puzzle. J Am Soc Nephrol 23: 1911–1913, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Gwinner W, Plasger J, Brandes RP, Kubat B, Schulze M, Regele H, Kerjaschki D, Olbricht CJ, Koch KM: Role of xanthine oxidase in passive Heymann nephritis in rats. J Am Soc Nephrol 10: 538–544, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Exner M, Susani M, Witztum JL, Hovorka A, Curtiss LK, Spitzauer S, Kerjaschki D: Lipoproteins accumulate in immune deposits and are modified by lipid peroxidation in passive Heymann nephritis. Am J Pathol 149: 1313–1320, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31.Neale TJ, Ojha PP, Exner M, Poczewski H, Rüger B, Witztum JL, Davis P, Kerjaschki D: Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest 94: 1577–1584, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neale TJ, Ullrich R, Ojha P, Poczewski H, Verhoeven AJ, Kerjaschki D: Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc Natl Acad Sci U S A 90: 3645–3649, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prunotto M, Carnevali ML, Candiano G, Murtas C, Bruschi M, Corradini E, Trivelli A, Magnasco A, Petretto A, Santucci L, Mattei S, Gatti R, Scolari F, Kador P, Allegri L, Ghiggeri GM: Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 21: 507–519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grune T, Reinheckel T, Davies KJ: Degradation of oxidized proteins in mammalian cells. FASEB J 11: 526–534, 1997 [PubMed] [Google Scholar]
- 35.Shringarpure R, Grune T, Davies KJ: Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci 58: 1442–1450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grune T, Davies KJ: The proteasomal system and HNE-modified proteins. Mol Aspects Med 24: 195–204, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ: The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J 432: 585–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doeppner TR, Mlynarczuk-Bialy I, Kuckelkorn U, Kaltwasser B, Herz J, Hasan MR, Hermann DM, Bahr M: The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 135[Pt 11]: 3282–3297, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R: Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol 22: 336–348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T: p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Giordano S, Zhang J: Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiken CT, Kaake RM, Wang X, Huang L: Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics 10: R110.006924, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X: Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest 121: 3689–3700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Powell SR, Wang X: Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. FASEB J 25: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ: Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40: 1087–1093, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Davies DJ, Brewer DB: Irreversible glomerular damage following heterologous serum albumin overload. J Pathol 123: 45–52, 1977 [DOI] [PubMed] [Google Scholar]
- 47.Weening JJ, Van Guldener C, Daha MR, Klar N, van der Wal A, Prins FA: The pathophysiology of protein-overload proteinuria. Am J Pathol 129: 64–73, 1987 [PMC free article] [PubMed] [Google Scholar]
- 48.Heymann W, Lund HZ, Hackel DB: The nephrotic syndrome in rats; with special reference to the progression of the glomerular lesion and to the use of nephrotoxic sera obtained from ducks. J Lab Clin Med 39: 218–224, 1952 [PubMed] [Google Scholar]
- 49.Salant DJ, Quigg RJ, Cybulsky AV: Heymann nephritis: Mechanisms of renal injury. Kidney Int 35: 976–984, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Salant DJ, Cybulsky AV: Experimental glomerulonephritis. Methods Enzymol 162: 421–461, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Kerjaschki D, Schulze M, Binder S, Kain R, Ojha PP, Susani M, Horvat R, Baker PJ, Couser WG: Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J Immunol 143: 546–552, 1989 [PubMed] [Google Scholar]
- 52.Meyer-Schwesinger C, Lange C, Bröcker V, Agustian P, Lehmann U, Raabe A, Brinkmeyer M, Kobayashi E, Schiffer M, Büsche G, Kreipe HH, Thaiss F, Becker JU: Bone marrow-derived progenitor cells do not contribute to podocyte turnover in the puromycin aminoglycoside and renal ablation models in rats. Am J Pathol 178: 494–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coers W, Huitema S, van der Horst ML, Weening JJ: Puromycin aminonucleoside and adriamycin disturb cytoskeletal and extracellular matrix protein organization, but not protein synthesis of cultured glomerular epithelial cells. Exp Nephrol 2: 40–50, 1994 [PubMed] [Google Scholar]
- 54.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Cohen CD, Frach K, Schlöndorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Cohen CD, Klingenhoff A, Boucherot A, Nitsche A, Henger A, Brunner B, Schmid H, Merkle M, Saleem MA, Koller KP, Werner T, Gröne HJ, Nelson PJ, Kretzler M: Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc Natl Acad Sci U S A 103: 5682–5687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen CD, Kretzler M: Gene expression analysis in microdissected renal tissue. Current challenges and strategies. Nephron 92: 522–528, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, Zahner G, Wolf G, Helmchen U, Schaerli P, Stahl RA, Thaiss F: Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol 17: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Meyer TN, Schwesinger C, Wahlefeld J, Dehde S, Kerjaschki D, Becker JU, Stahl RA, Thaiss F: A new mouse model of immune-mediated podocyte injury. Kidney Int 72: 841–852, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Abramoff M, Ram SJ: Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
- 62.Ehlers MD: Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6: 231–242, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW: A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest 107: 853–860, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vivekanandan-Giri A, Byun J, Pennathur S: Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol 491: 73–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahlff J, Trusch M, Haag F, Bacher U, Horst A, Schlüter H, Binder M: Antigen-specificity of oligoclonal abnormal protein bands in multiple myeloma after allogeneic stem cell transplantation. Cancer Immunol Immunother 61: 1639–1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.