Abstract
The length polymorphism of guanosine thymidine dinucleotide repeats in the heme oxygenase-1 gene promoter is associated with cardiovascular events and mortality in high-risk populations. Experimental data suggest that heme oxygenase-1 protects against kidney disease. However, the association between this polymorphism and long-term risk of CKD in high-risk patients is unknown. We analyzed the allelic frequencies of guanosine thymidine dinucleotide repeats in the heme oxygenase-1 gene promoter in 386 patients with coronary artery disease recruited from January 1999 to July 2001 and followed until August 31, 2012. The S allele represents short repeats (<27), and the L allele represents long repeats (≥27). The primary renal end points consisted of sustained serum creatinine doubling and/or ESRD requiring long-term RRT. The secondary end points were major adverse cardiovascular events and mortality. At the end of study, the adjusted hazard ratios (95% confidence intervals) for each L allele in the additive model were 1.99 (1.27 to 3.14; P=0.003) for the renal end points, 1.70 (1.27 to 2.27; P<0.001) for major adverse cardiovascular events, and 1.36 (1.04 to 1.79; P=0.03) for mortality. With cardiac events as time-dependent covariates, the adjusted hazard ratio for each L allele in the additive model was 1.91 (1.20 to 3.06; P=0.01) for the renal end points. In conclusion, a greater number of guanosine thymidine dinucleotide repeats in the heme oxygenase-1 gene promoter is associated with higher risk for CKD, cardiovascular events, and mortality among patients with coronary artery disease.
Keywords: heme oxygenase-1, polymorphism, coronary artery disease, CKD, guanosine thymidine dinucleotide, mortality
Heme oxygenase (HO) is the rate-limiting enzyme in heme degradation. The enzyme generates free iron, biliverdin, and carbon monoxide. Biliverdin is subsequently converted to bilirubin by biliverdin reductase, and free iron is rapidly sequestered by ferritin.1 HO is a cytoprotective enzyme that potentially exerts antioxidant, anti-inflammatory, and antiapoptotic functions.2 HO-1 is the inducible isoform, whereas HO-2 is constitutively expressed. HO-1 is expressed in various tissues and is upregulated by cellular stress. HO-1 is known to be protective against atherosclerosis, hepatic ischemia-reperfusion injury, hyperoxia-induced lung injury, and corneal inflammation in experimental studies.3–6
The human HO-1 gene has been mapped to chromosome 22q12 and the number of guanosine thymidine dinucleotide [(GT)n] repeats in the HO-1 gene microsatellite promoter is inversely associated with HO-1 mRNA levels and enzyme activity.7,8 Previous studies have demonstrated an increased susceptibility to cardiovascular events and increased mortality of longer (GT)n repeats in the HO-1 gene promoter in high-risk populations such as patients with diabetes mellitus, hypercholesterolemia, a history of smoking, peripheral artery disease, or arsenic exposure.8–16 Our recent study also showed that longer (GT)n repeats in the HO-1 gene promoter were associated with a higher risk of long-term cardiovascular events and mortality in hemodialysis patients.17 Until now, the investigations for this polymorphism were mostly restricted to graft survival in kidney transplant recipients.18 The effect of the length polymorphism in the HO-1 gene promoter on the risk of CKD is a largely undefined body of knowledge.
Coronary artery disease (CAD) per se is a major independent risk factor for subsequent development and progression of CKD.19–21 Kiyosue et al. found that the deterioration of renal function was correlated with the severity of CAD in Japanese patients.20 We recently revealed that the GFR declined faster in CAD than that in normal coronary artery of Taiwanese patients.21 Longer (GT)n repeats in the HO-1 gene promoter associated with long-term outcomes of CAD, such as major adverse cardiovascular events, target vessel revascularization, and overall mortality8–12; however, the relevance of this polymorphism in native kidney function of patients with CAD is unknown. The protective effect of HO-1 against kidney injury is well known in a variety of animal models.2,18,22–26 We therefore performed an association study in patients with CAD to investigate whether the length polymorphism of the HO-1 gene promoter is associated with the risk of CKD.
Results
Initially, 402 patients with CAD were enrolled from a tertiary hospital. During a median follow-up period of 10.2 years (interquartile range, 6.31−11.6), 16 patients were excluded from the study because of a follow-up time of <6 months (n=3) and insufficient creatinine follow-up data (n=13). Finally, 386 patients with CAD with a mean age of 70 years were analyzed.
Length Polymorphism of (GT)n Repeats in the HO-1 Gene Promoter
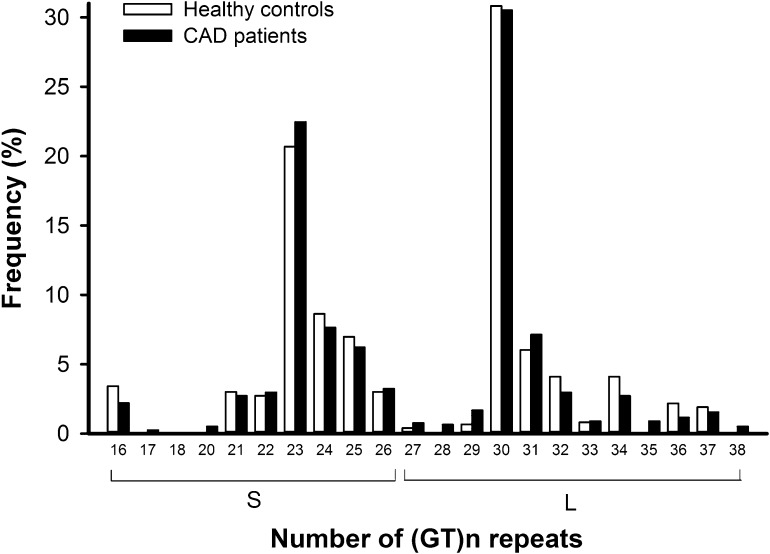
Figure 1 shows the frequency distribution of (GT)n repeats of the HO-1 microsatellite promoter in the 386 patients with CAD and 361 healthy controls. The allelic distribution ranged from 16 to 38 GT repeats, with 23 and 30 GT repeats being the two most common alleles. We chose 27 GT repeats as a cutoff to classify the participants for allele typing, and the cutoff value was consistent with the previously published literature.10,14,17,27 Thus, short repeats with <27 GT repeats were designated as S alleles, and long repeats with at least 27 GT repeats were designated as L alleles. Accordingly, the participants were categorized into S/S, S/L, or L/L genotypes. In Table 1, the genotype proportions and L-allelic frequencies of HO-1 polymorphism in patients with CAD were comparable with those in healthy controls and Hardy–Weinberg equilibrium was met. In this study, all participants were Taiwanese and had similar ethnic backgrounds. Therefore, statistical artifacts caused by population stratification could be ruled out as described by Pritchard and Rosenberg.28
Figure 1.
Frequency distribution of the number of (GT)n repeats of the heme oxygenase-1 microsatellite promoter. There are 386 patients with CAD and 361 healthy controls. The (GT)n repeats range from 16 to 38. There is a bimodal distribution with one peak located at 23 repeats and the other located at 30 repeats in both groups. The frequency distributions of (GT)n repeats are not significantly different between patients with CAD and healthy controls.
Table 1.
Genotype and allelic frequency of HO-1 microsatellite promoter polymorphism in healthy controls and patients with CAD
| Healthy Controls (n=361) | Patients with CAD (n=386) | |
|---|---|---|
| Genotype, n (%) | ||
| S/S | 78 (21.6) | 94 (24.4) |
| S/L | 194 (53.7) | 187 (48.4) |
| L/L | 89 (24.7) | 105 (27.2) |
| L-allelic frequency | 0.52 | 0.51 |
S allele, number of (GT)n repeats <27; L allele, number of (GT)n repeats ≥27.
Baseline Demographic and Laboratory Data
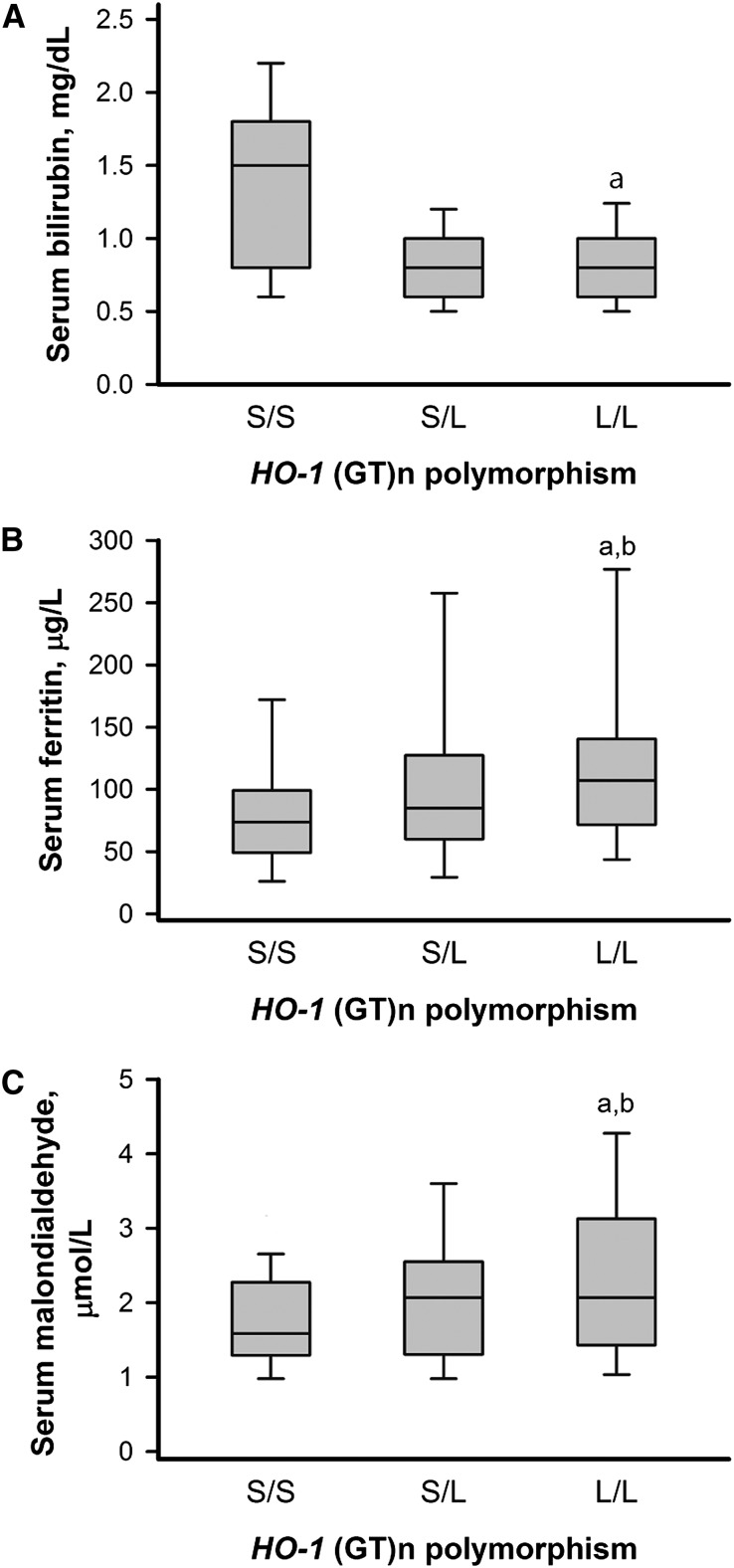
Patients with CAD among the three genotypes of HO-1 promoter polymorphism had no significant differences in age at entry, sex, comorbidity, LDL and HDL cholesterol levels, and smoking status (Table 2). No significant differences across genotype groups were found in the baseline eGFR, proteinuria, use of statins or renin-angiotensin system blockades, serum albumin, calcium, phosphorus, and hemoglobin levels. Intriguingly, the serum bilirubin level was lower in L allele carriers (L/L and S/L genotypes) and higher in patients with the S/S genotype (Figure 2). Serum ferritin and malondialdehyde levels were highest in the L/L genotype and lower in the S/L and S/S genotypes (Figure 2).
Table 2.
Baseline demographic characteristics and laboratory data of patients with CAD stratified by genotypes of HO-1 microsatellite promoter polymorphism
| Characteristic | HO-1 Promoter Genotype | ||||
|---|---|---|---|---|---|
| All Patients (n=386) | S/S (n=94) | S/L (n=187) | L/L (n=105) | P Value | |
| Age, yr | 70±8 | 71±8 | 70±8 | 71±8 | 0.76 |
| Men, % | 92.7 | 93.6 | 92.5 | 92.4 | 0.93 |
| Current smoker, % | 33.1 | 36.7 | 30.3 | 34.7 | 0.53 |
| Comorbidities, % | |||||
| Hypertension | 73.8 | 80.9 | 73.3 | 68.6 | 0.14 |
| Diabetes mellitus | 33.7 | 30.9 | 34.8 | 34.3 | 0.80 |
| Congestive heart failure | 12.4 | 11.7 | 11.8 | 14.3 | 0.80 |
| Cerebrovascular disease | 6.5 | 4.3 | 5.3 | 10.5 | 0.14 |
| Peripheral artery disease | 3.4 | 5.3 | 1.1 | 5.7 | 0.05 |
| Total no. of antihypertensive medications, % | 2.7±1.6 | 2.7±1.4 | 2.5±1.5 | 3.1±1.7 | 0.75 |
| Renin-angiotensin system blockades, % | 45.6 | 45.7 | 44.9 | 46.6 | 0.96 |
| Statins, % | 23.8 | 24.5 | 23.5 | 23.8 | 0.99 |
| Laboratory parameters | |||||
| Creatinine, mg/dl | 1.3±0.4 | 1.3±0.4 | 1.2±0.3 | 1.3±0.6 | 0.22 |
| eGFR, ml/min per 1.73 m2 | 64±17 | 63±17 | 65±15 | 63±19 | 0.44 |
| Proteinuria, % | 12.2 | 12.1 | 13.4 | 10.1 | 0.71 |
| Albumin, g/dl | 4.1±0.4 | 4.0±0.4 | 4.1±0.4 | 4.1±0.4 | 0.29 |
| Total cholesterol, mg/dl | 187±37 | 193±41 | 183±32 | 188±40 | 0.08 |
| LDL cholesterol, mg/dl | 122±27 | 125±31 | 121±26 | 121±27 | 0.46 |
| HDL cholesterol, mg/dl | 38±8 | 38±8 | 37±8 | 39±8 | 0.12 |
| Triglyceride, mg/dl | 151±118 | 157±103 | 143±87 | 159±168 | 0.46 |
| Fasting serum glucose, mg/dl | 116±46 | 114±38 | 115±46 | 119±51 | 0.64 |
| Uric acid, mg/dl | 7.3±1.9 | 7.4±1.7 | 7.1±2.0 | 7.3±1.8 | 0.51 |
| Calcium, mg/dl | 9.0±0.5 | 9.0±0.6 | 8.9±0.4 | 9.0±0.5 | 0.77 |
| Phosphorus, mg/dl | 3.4±0.6 | 3.5±0.5 | 3.4±0.6 | 3.4±0.7 | 0.55 |
| Hemoglobin, g/dl | 13.2±1.5 | 13.3±1.7 | 13.1±1.5 | 13.3±1.5 | 0.43 |
| Iron, mg/dl | 82±98 | 69±72 | 83±90 | 91±129 | 0.27 |
| Transferrin saturation, % | 26±14 | 25±13 | 26±13 | 28±15 | 0.26 |
Figure 2.
Levels of serum bilirubin, ferritin, and malondialdehyde by genotypes. Baseline serum bilirubin (A), ferritin (B), and malondialdehyde (C) in 386 patients with CAD stratified by the genotype of the HO-1 microsatellite promoter polymorphism. Whisker plots show the 10th, 25th, 50th, 75th, and 90th percentiles of distribution. aP<0.05 versus S/S genotype; bP<0.05 versus S/L genotype.
Risk of CKD
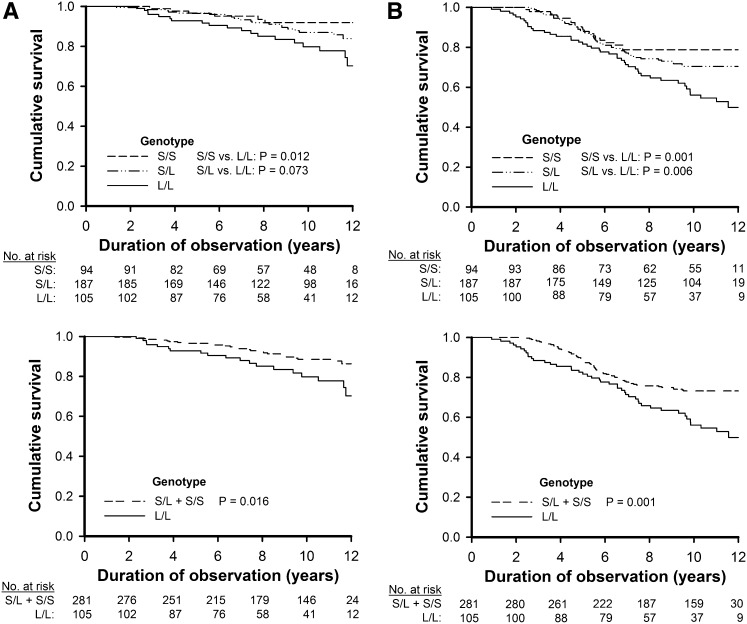
At the end of study, 47 patients (12.2%) reached the renal end points, which included sustained serum creatinine doubling and ESRD necessitating long-term RRT. In this study, we used the composite renal end points to evaluate the risk of CKD. To ensure the events were CKD but not AKI, the patients followed-up for fewer than 6 months were ruled out. Data on at least two follow-up creatinine measurements were obtained. The events of creatinine doubling were restricted to sustained high creatinine levels; this means that if the creatinine level regressed to lower than the doubling value during follow-up, the renal end point was not reached. ESRD is defined as necessitating long-term RRT but not for a short temporary period. Moreover, acute elevation of creatinine and dialysis starting before death were assumed to be AKI and were not included in the renal end points. Kaplan–Meier analysis curves among 386 patients with CAD showed that the risk of the renal end points was significantly higher in the L/L genotype than in the S/S genotype (P=0.01) and S allele carriers (S/L and S/S genotypes) (P=0.02) (Figure 3A). When we assessed the association of this polymorphism with each outcome, there were no major differences between the S/S and S/L genotype groups for all three types of cumulative events (Table 3). This implies that a recessive model would best fit the observed data. In the recessive model of a Cox regression analysis, individuals with the L/L genotype had approximately 2.4-fold the risk for the renal end points as the S allele carriers, which led to an adjusted hazard ratio (HR) of 2.43 (95% confidence interval [95% CI], 1.28 to 2.04; P=0.01) (Table 3). In the additive model, each L allele had an adjusted HR of 1.99 (95% CI, 1.27 to 3.14; P=0.003) for the renal end points (Table 3). We further validated that the risk of CKD is independent to the risk of cardiac outcomes that developed during follow-up using the cardiac events as time-dependent covariates in the Cox regression analysis (Table 4). The L/L genotype led to an adjusted HR of 2.41 (95% CI, 1.31 to 4.55; P=0.01) for the renal end points as the S allele carriers, and each L allele had an adjusted HR of 1.91 (95% CI, 1.20 to 3.06; P=0.01) for the renal end points (Table 4).
Figure 3.
Survival curves for renal end points and all-cause mortality by genotypes. Kaplan–Meier cumulative survival curves for renal end points (A) and all-cause mortality (B) among the 386 patients with CAD in relation to the genotype of the HO-1 microsatellite promoter polymorphism. The upper panels are the three genotypes (S/S, S/L, and L/L), and the lower panels are the genotypes in a recessive model (S/L+S/S and L/L).
Table 3.
Cumulative renal, major adverse cardiovascular and mortality events, and HRs (95% CIs) of genotypes of the HO-1 microsatellite promoter polymorphism in patients with CAD with a median follow-up of 10.2 years
| End Point | Cumulative Events per 1000 Person-Years by Genotype | Cox Regression Modelsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S/S | S/L | L/L | Recessive Model for L/L versus S/L+S/S | Additive Model for Each L Allele | |||||||
| Crude HR (95% CI) | Crude P Value | Adjusted HR (95% CI) | Adjusted P Value | Crude HR (95% CI) | Crude P Value | Adjusted HR (95% CI) | Adjusted P Value | ||||
| Serum creatinine doubling and/or ESRD | 7.4 | 13.1 | 22.1 | 2.02 (1.13 to 3.62) | 0.02 | 2.43 (1.28 to 2.04) | 0.01 | 1.74 (1.14 to 2.66) | 0.01 | 1.99 (1.27 to 3.14) | 0.003 |
| Major adverse cardiovascular events | 20.2 | 25.8 | 56.2 | 2.33 (1.57 to 3.46) | <0.001 | 2.05 (1.36 to 3.11 | 0.001 | 1.77 (1.33 to 2.37) | <0.001 | 1.70 (1.27 to 2.27) | <0.001 |
| All-cause mortality | 22.0 | 30.7 | 53.4 | 1.87 (1.29 to 2.71) | 0.001 | 1.47 (1.02 to 2.18) | 0.01 | 1.58 (1.22 to 2.06) | 0.001 | 1.36 (1.04 to 1.79) | 0.03 |
A multivariate Cox regression model was adjusted for age, sex, smoking status, diabetes, hypertension, prior congestive heart failure, stroke or peripheral arterial disease, total cholesterol, HDL cholesterol, serum albumin, hemoglobin, eGFR, presence of proteinuria at baseline, and use of renin-angiotensin system blockades or statins.
Table 4.
Adjusted HRs (95% CIs) of genotypes of the HO-1 microsatellite promoter polymorphism for renal end pointsa in patients with CAD with a median follow-up of 10.2 years
| Model | Recessive Model for L/L versus S/L+S/S | Additive Model for Each L Allele | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Cox regressionb | 2.43 (1.28 to 2.04) | 0.01 | 1.99 (1.27 to 3.14) | 0.003 |
| Cox regression with time-varying covariatec | 2.41 (1.31 to 4.55) | 0.01 | 1.91 (1.20 to 3.06) | 0.01 |
Renal end points are serum creatinine doubling and/or ESRD necessitating long-term RRT.
A Cox regression model was adjusted for age, sex, smoking status, diabetes, hypertension, prior congestive heart failure, stroke or peripheral arterial disease, total cholesterol, HDL cholesterol, serum albumin, hemoglobin, eGFR, presence of proteinuria at baseline, and use of renin-angiotensin system blockades or statins.
The multivariate Cox regression model was further analyzed using the cardiac events as the time-dependent covariates.
Major Adverse Cardiovascular Events and All-Cause Mortality
Of the study patients, 101 (26.4%) developed major adverse cardiovascular events and 117 (30.3%) died. Of the deceased patients, 42 (35.9%) died from cardiovascular causes. In the Kaplan–Meier curve analyses, the risk of all-cause mortality was significantly higher in the L/L genotype than in the S/S genotype (P=0.001) and the S/L genotype (P=0.01) (Figure 3B). The S allele noncarriers had a significantly higher risk of mortality than the S allele carriers (S/L and S/S genotypes) (P=0.001) (Figure 3B). In the recessive model, patients with the L/L genotype had approximately 2-fold the risk for major adverse cardiovascular events and 1.5-fold the risk for death as the S allele carriers, which resulted in adjusted HRs of 2.05 (95% CI, 1.36 to 3.11; P=0.001) and 1.47 (95% CI, 1.02 to 2.18; P=0.01), respectively (Table 3). In the additive model, each L allele had adjusted HRs of 1.70 (95% CI, 1.27 to 2.27; P<0.001) for major adverse cardiovascular events and 1.36 (95% CI, 1.04 to 1.79; P=0.03) for death, respectively (Table 3).
Discussion
This study, with a median follow-up of 10.2 years among 386 patients with CAD, is the first to demonstrate that there is a significantly increased risk of CKD for patients with the L/L genotype in HO-1 gene promoter polymorphism. Individuals with this genotype had approximately 2.4-fold the risk for sustained serum creatinine doubling and/or ESRD necessitating long-term RRT of those with S/S or S/L genotypes. The significant association of this polymorphism with cardiovascular outcome and mortality in patients with CAD also corroborated previous studies in these high-risk populations.8–16
Ample evidence has shown the beneficial effect of HO-1 in experimental kidney disease, including ischemia-reperfusion kidney injury, nephrotoxin or angiotensin II–induced renal injury, kidney transplantation, and diabetic models.2,18,22 Kim et al.23 demonstrated the renoprotective effect of HO-1 using its inducer and inhibitor through an antiapoptotic pathway in the unilateral ureteral obstruction (UUO) model. Kie et al.24 showed that HO-1 deficiency promoted epithelial-mesenchymal transition and renal fibrosis in the UUO model in HO-1 knockout mice. Desbuards et al.25 used hemin, an inducer of HO-1, to attenuate interstitial fibrosis in 5/6 nephrectomized rats via inhibiting TGF-β and caspase-3 expression and increasing bone morphogenetic protein-7 expression. Correa-Costa et al.26 further revealed that pretreatment or delayed treatment with hemin both reduced renal dysfunction and decreased the expression of proinflammatory molecules, fibrosis-related molecules, and collagen deposition in rats subjected to UUO. In addition, HO-2, which contributes the bulk of HO activity in the unstressed kidney, was also suspected to play a protective role in the animal kidney models.29,30 However, the association between the HO-2 gene polymorphism and kidney disease in humans is still obscure.
The mechanism of HO-1 protection in the kidney is related to heme degradation because a large amount of free heme is pro-oxidant, proinflammatory, and proapoptotic and HO-1 is responsible for regulating its cellular levels. HO-1 also exerts protective effects through its reactive products, bilirubin and carbon monoxide.2,18,22 Bilirubin has both antioxidant and anti-inflammatory properties.31,32 In humans, the length of (GT)n repeats in the HO-1 gene promoter is inversely associated with HO-1 mRNA levels and enzyme activity.8 Because HO-1 is a rate-limiting enzyme for bilirubin production, the serum bilirubin concentration is determined in part by this length polymorphism. Our study confirmed previous findings that the L allele was significantly associated with lower serum bilirubin,33 and the gene explained a large proportion of the variation in bilirubin levels. We further found that patients with the L/L genotype had less ability against oxidative stress and inflammation, which was exhibited by higher levels of serum malondialdehyde and ferritin. The results corroborated previous studies.9,11,33 Although ferritin increases in response to oxidative damage and inflammation and is considered an inflammation marker, ferritin has been shown to have antioxidant properties through iron sequestering and the potential protective effects in the kidney.34 Being a potential cytoprotective protein and an inflammation marker at the same time, the mechanisms by which HO-1 polymorphism confers the variance in ferritin values remain to be elucidated. In this study, the Cox regression with time-varying covariate analyses (Supplemental Table 1) revealed that lower total bilirubin was significantly associated with the risk of CKD progression. Our additional data suggest that the association of the L/L genotype or L allele with the risk of CKD is at least in part mediated by the intermediate phenotype.
Evidence for causality between inflammation-oxidative stress and renal function progression may be possible using a Mendelian randomization approach.35 For example, S alleles result in higher HO-1 expressions of life-long persistence. Therefore, a higher protective effect against renal function decline from these alleles can be expected. The association between the polymorphism with clinical outcomes is less likely to be influenced by reverse causation or confounding.
Human studies assessing the length polymorphism of the HO-1 gene promoter and renal outcome are mostly restricted to graft survival in kidney transplant recipients and the results are conflicting.18 Only two cross-sectional studies examined the relationship of this polymorphism in CKD. Courtney et al.36 found that the HO-1 genotype conferred no significant influence on the mean age at commencement of RRT among patients with autosomal dominant polycystic kidney disease or IgA nephropathy. Chin et al.37 found that the longer length of the HO-1 gene promoter was related to an eGFR<60 ml/min per 1.73 m2 at diagnosis of IgA nephropathy. The discrepancy between our study and that by Courtney et al. may be mainly because the milieu of factors linked to the progression of CKD were not adjusted in the study by Courtney et al. In addition, differences in study populations (autosomal dominant polycystic kidney disease or IgA nephropathy versus CAD), genotype distribution, sample size, and study design (cross-sectional analysis versus longitudinal follow-up) are also noted. To our knowledge, our study is the first longitudinal long-term study to investigate HO-1 promoter polymorphism and risk of CKD.
Given the estimated allele frequency and its effect on serum bilirubin, the L allele of the HO-1 gene promoter is a likely candidate for the reported trait. If approximately 27% of the CAD population has a strong adverse effect in renal function decline because of the HO-1 length polymorphism, it would have significant implications in future CKD prevention for the high-risk population.
Some limitations in this study should be acknowledged. First, this is a single-center study in patients with CAD in the Taiwanese population and confirmatory replication studies in other centers and other populations are needed. Second, the creatinine follow-up interval was not regular in usual clinical care instead of regular study visits, and the creatinine doubling time might be underdiagnosed. Finally, our study was observational in nature, so it cannot prove causality. However, to ensure adequate statistical power (α=0.05; 1−β=0.8; no loss of follow-up), at least 364 patients with CAD should be enrolled to examine a relative risk increment of 40%. Apart from impracticability to conduct a randomized controlled trial, emerging evidence38 suggests that well designed observational studies could yield comparable outcomes.
In conclusion, this prospective cohort study shows that homozygote L allele carriers exhibited a strong association with higher risk of CKD, major adverse cardiovascular events, and overall mortality among patients with CAD. Further studies are required to validate this association.
Concise Methods
Research Participants
Study participants were recruited from January 1999 to July 2001 at a tertiary hospital in Taipei, a metropolitan city that is not the arseniasis-endemic area in Taiwan. A total of 419 patients undergoing coronary angiography agreed to participate in this study. Seventeen patients were excluded from the study because of non-CAD (n=4), insignificant CAD (n=11), history of receiving a renal transplant (n=1), and ESRD undergoing dialysis at entry (n=1). CAD was documented by angiographic evidence of at least ≥75% stenosis of at least one major coronary artery or a history of prior angioplasty, coronary artery bypass surgery, or myocardial infarction by history validated by electrocardiographic changes. During follow-up, patients who had only one or no follow-up creatinine data and who were followed-up for fewer than 6 months were also excluded from the study. A total of 386 patients with CAD were enrolled. A group of 361 healthy controls were also recruited from volunteers who were receiving health checkups. The control patients were enrolled for genotyping of the length polymorphism of (GT)n repeats in the HO-1 gene promoter. The healthy volunteers had no CAD or cardiovascular or kidney diseases. The study protocol was approved by the institutional review board of Taipei Tzuchi Hospital. Informed consent was obtained from all participants, and our study complies with the Declaration of Helsinki.
Clinical Data Collection
Baseline demographic data were recorded at the time of recruitment. Hypertension was defined as a measured systolic BP>140 mmHg, a diastolic BP>90 mmHg, and/or the use of antihypertensive medications. Diabetes was diagnosed on the basis of the World Health Organization criteria. Congestive heart failure was diagnosed as a left ventricular ejection fraction <40% by echocardiography and symptoms/signs of clinical criteria. Cerebrovascular disease was diagnosed as a brain infarction or hemorrhage by brain imaging and clinical symptoms/signs. Peripheral artery disease was diagnosed as an ankle-brachial index ≤0.9 and clinical symptoms/signs.
Laboratory Measurements
Venous blood samples were drawn from the patients who had fasted overnight. Albumin was measured using the bromocresol green method. Iron, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, glucose, calcium, phosphorus, and creatinine levels in the serum were determined using commercial kits and a Hitachi 7600 autoanalyzer (Roche Modular; Hitachi, Ltd., Tokyo, Japan). The total iron binding capacity (TIBC) was measured using the TIBC Microtest (Daiichi, Tokyo, Japan), and serum ferritin was determined using an RIA (Incstar, Stillwater, MN). Transferrin saturation was calculated as the ratio of serum iron to TIBC and was presented as percentages. Proteinuria was defined as a value of trace or higher on a urine dipstick assay (Clinitek Atlas 10; Bayer HealthCare, Mishawaka, IN). Plasma malondialdehyde was determined with a thiobarbituric acid test. The adducts consisting of two molecules of thiobarbituric acid were separated by an HPLC method described by Nielsen et al.39 Total serum bilirubin was measured using the metavanadate oxidation method (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Length Polymorphism of (GT)n Repeats in the HO-1 Gene Promoter
Genomic DNAs were extracted from leukocytes with conventional procedures. The 5′-flanking region containing (GT)n repeats of the HO-1 gene was amplified by the PCR with a 5-carboxyfluorescein–labeled sense primer (5′-AGAGCCTGCAGCTTCTCAGA-3′) and an antisense primer (5′-ACAAAGTCTGGCCATAGGAC-3′), according to a previously published procedure.40 The PCR products were mixed together with the GenoType TAMRA DNA ladder (size range, 50–500 bp; GibcoBRL) and analyzed with an automated DNA sequencer (ABI Prism 377). Each size of the (GT)n repeats was calculated with GeneScan analysis software (PE Applied Biosystems).
Outcome Data Collection
The primary renal end points included sustained serum creatinine doubling and/or ESRD necessitating long-term RRT from the time of inclusion in the study. The secondary end points were major adverse cardiovascular events and all-cause mortality. The cohort was followed until August 31, 2012. The patients were followed-up for at least 6 months. A trained physician who was blinded to the length polymorphism of (GT)n repeats in the HO-1 gene promoter independently obtained information about the occurrence of interim ESRD, major adverse cardiovascular events, and cause of death by reviewing hospital records and making phone calls to the study patients. ESRD means the patients undergo long-term RRT, including hemodialysis, peritoneal dialysis, or renal transplantation. Major adverse cardiovascular events consist of myocardial infarction and ischemic stroke. All-cause mortality included cardiovascular death and noncardiovascular causes comprising infection, sepsis, malignancy, gastrointestinal bleeding, chronic obstructive lung disease, and chronic liver disease.
Statistical Analyses
A comparison of the genotypes and allelic frequencies of the HO-1 microsatellite promoter polymorphism between patients with CAD and healthy individuals was performed using a chi-squared test. Baseline descriptive variables were expressed as percentages for categorical data, means±SDs for continuous data with a normal distribution, and medians (interquartile ranges) for continuous data without a normal distribution. Potential differences among the three patient groups of the HO-1 promoter genotype were assessed with ANOVA for normally distributed data, the Kruskal–Wallis test for non-normally distributed data, or the Pearson chi-squared test for categorical variables. Cumulative survival curves for the renal end points and all-cause mortality were generated using the Kaplan–Meier method. Between-group survival rates among the genotypes of the HO-1 promoter polymorphism were compared using a log-rank test. The assumption of proportional hazards was confirmed by a log minus log plot and was met in the Cox models. A multivariate Cox regression model was used to estimate the HRs of the renal end points, major adverse cardiovascular events, and all-cause mortality in relation to the genotypes of the HO-1 microsatellite promoter polymorphism. The analysis was adjusted for age, sex, smoking status, diabetes, hypertension, prior congestive heart failure, stroke, and peripheral artery disease, total cholesterol, HDL cholesterol, serum albumin, hemoglobin, eGFR, presence of proteinuria at baseline, and use of renin-angiotensin system blockades and statins. Because the length polymorphism in the HO-1 promoter was significantly associated with bilirubin, ferritin, and malondialdehyde, these three variables were not offered simultaneously in a Cox regression model to avoid multicollinearity. The HRs of the renal end points were further examined using cardiac events as time-dependent covariates in the Cox regression model in relation to the genotypes of the HO-1 microsatellite promoter polymorphism, total bilirubin, serum ferritin, and malondialdehyde, respectively. Statistical analysis was performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL). All P values were two-tailed. P values<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank P.C. Lee for her expert secretarial assistance and graphic design.
This study was supported by grants from the National Science Council (NSC 99-2314-B-010-002-MY3 and NSC 102-2314-B-010-004-MY3), the Taipei Veterans General Hospital (V96ER2-012 and V99C1-121), and the Ministry of Education’s Aim for the Top University Plan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111205/-/DCSupplemental.
References
- 1.Maines MD: Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2: 2557–2568, 1988 [PubMed] [Google Scholar]
- 2.Nath KA: Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY: Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 104: 1519–1525, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Tamura T, Kondo T, Ogawa K, Fukunaga K, Ohkohchi N: Protective effect of heme oxygenase-1 on hepatic ischemia-reperfusion injury through inhibition of platelet adhesion to the sinusoids. J Gastroenterol Hepatol 28: 700–706, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM: Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 103: 1047–1054, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laniado-Schwartzman M, Abraham NG, Conners M, Dunn MW, Levere RD, Kappas A: Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem Pharmacol 53: 1069–1075, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Kutty RK, Kutty G, Rodriguez IR, Chader GJ, Wiggert B: Chromosomal localization of the human heme oxygenase genes: Heme oxygenase-1 (HMOX1) maps to chromosome 22q12 and heme oxygenase-2 (HMOX2) maps to chromosome 16p13.3. Genomics 20: 513–516, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O: Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther 8: 433–440, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY: Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, Aizawa T, Ishizaka N, Nagai R: Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol 22: 1680–1685, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Schillinger M, Exner M, Minar E, Mlekusch W, Müllner M, Mannhalter C, Bach FH, Wagner O: Heme oxygenase-1 genotype and restenosis after balloon angioplasty: A novel vascular protective factor. J Am Coll Cardiol 43: 950–957, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Chau LY, Lin MW, Chen LC, Yo MH, Chen JW, Lin SJ: Heme oxygenase-1 gene promotor microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J 25: 39–47, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dick P, Schillinger M, Minar E, Mlekusch W, Amighi J, Sabeti S, Schlager O, Raith M, Endler G, Mannhalter C, Wagner O, Exner M: Haem oxygenase-1 genotype and cardiovascular adverse events in patients with peripheral artery disease. Eur J Clin Invest 35: 731–737, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Wu MM, Chiou HY, Chen CL, Wang YH, Hsieh YC, Lien LM, Lee TC, Chen CJ: GT-repeat polymorphism in the heme oxygenase-1 gene promoter is associated with cardiovascular mortality risk in an arsenic-exposed population in northeastern Taiwan. Toxicol Appl Pharmacol 248: 226–233, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Wu MM, Chiou HY, Lee TC, Chen CL, Hsu LI, Wang YH, Huang WL, Hsieh YC, Yang TY, Lee CY, Yip PK, Wang CH, Hsueh YM, Chen CJ: GT-repeat polymorphism in the heme oxygenase-1 gene promoter and the risk of carotid atherosclerosis related to arsenic exposure. J Biomed Sci 17: 70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MM, Chiou HY, Chen CL, Hsu LI, Lien LM, Wang CH, Hsieh YC, Wang YH, Hsueh YM, Lee TC, Cheng WF, Chen CJ: Association of heme oxygenase-1 GT-repeat polymorphism with blood pressure phenotypes and its relevance to future cardiovascular mortality risk: An observation based on arsenic-exposed individuals. Atherosclerosis 219: 704–708, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Chen YH, Hung SC, Tarng DC: Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin J Am Soc Nephrol 8: 1756–1763, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courtney AE, Maxwell AP: Heme oxygenase 1: Does it have a role in renal cytoprotection? Am J Kidney Dis 51: 678–690, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kiyosue A, Hirata Y, Ando J, Fujita H, Morita T, Takahashi M, Nagata D, Kohro T, Imai Y, Nagai R: Relationship between renal dysfunction and severity of coronary artery disease in Japanese patients. Circ J 74: 786–791, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Hsu TW, Kuo KL, Hung SC, Huang PH, Chen JW, Tarng DC: Progression of kidney disease in non-diabetic patients with coronary artery disease: Predictive role of circulating matrix metalloproteinase-2, -3, and -9. PLoS ONE 8: e70132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Nick HS: Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Yang JI, Jung MH, Hwa JS, Kang KR, Park DJ, Roh GS, Cho GJ, Choi WS, Chang SH: Heme oxygenase-1 protects rat kidney from ureteral obstruction via an antiapoptotic pathway. J Am Soc Nephrol 17: 1373–1381, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kie JH, Kapturczak MH, Traylor A, Agarwal A, Hill-Kapturczak N: Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desbuards N, Hyvelin JM, Machet MC, Eder V, Garrigue MA, Halimi JM, Antier D: Heme oxygenase-1 inducer hemin attenuates the progression of remnant kidney model. Nephron, Exp Nephrol 113: e35–e44, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Correa-Costa M, Semedo P, Monteiro AP, Silva RC, Pereira RL, Gonçalves GM, Marques GD, Cenedeze MA, Faleiros AC, Keller AC, Shimizu MH, Seguro AC, Reis MA, Pacheco-Silva A, Câmara NO: Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS ONE 5: e14298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lüblinghoff N, Winkler K, Winkelmann BR, Seelhorst U, Wellnitz B, Boehm BO, März W, Hoffmann MM: Genetic variants of the promoter of the heme oxygenase-1 gene and their influence on cardiovascular disease (the Ludwigshafen Risk and Cardiovascular Health study). BMC Med Genet 10: 36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard JK, Rosenberg NA: Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet 65: 220–228, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, Turkseven S, Lianos EA, Dennery PA, Abraham NG: Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction. J Am Soc Nephrol 17: 1073–1081, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Nath KA, Grande JP, Farrugia G, Croatt AJ, Belcher JD, Hebbel RP, Vercellotti GM, Katusic ZS: Age sensitizes the kidney to heme protein-induced acute kidney injury. Am J Physiol Renal Physiol 304: F317–F325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN: Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Willis D, Moore AR, Frederick R, Willoughby DA: Heme oxygenase: A novel target for the modulation of the inflammatory response. Nat Med 2: 87–90, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Chen YH, Chau LY, Chen JW, Lin SJ: Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care 31: 1615–1620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A: Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katan MB: Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1: 507–508, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Courtney AE, McNamee PT, Heggarty S, Middleton D, Maxwell AP: Association of functional haem oxygenase-1 gene promoter polymorphism with polycystic kidney disease and IgA nephropathy. Nephrol Dial Transplant 23: 608–611, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Chin HJ, Cho HJ, Lee TW, Na KY, Yoon HJ, Chae DW, Kim S, Jeon US, Do JY, Park JW, Yoon KW, Shin YT, Lee KW, Na KR, Cha DR, Kang YS, Progressive REnal disease and Medical Informatics and gEnomics Research (PREMIER) members : The heme oxygenase-1 genotype is a risk factor to renal impairment of IgA nephropathy at diagnosis, which is a strong predictor of mortality. J Korean Med Sci 24[Suppl]: S30–S37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Concato J, Shah N, Horwitz RI: Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342: 1887–1892, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P: Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem 43: 1209–1214, 1997 [PubMed] [Google Scholar]
- 40.Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, Sasaki H, Higuchi S, Okita N, Takase S, Saito H, Takahashi K, Shibahara S: Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet 100: 145–147, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.