Abstract
The mechanisms of mechanosensitivity underlying the response of the human bladder to stretch are poorly understood. Animal data suggest that stretch-activated two-pore-domain (K2P) K+ channels play a critical role in bladder relaxation during the filling phase. The objective of this study was to characterize the expression and function of stretch-activated K2P channels in the human bladder and to clarify their physiological role in bladder mechanosensitivity. Gene and protein analysis of the K2P channels TREK-1, TREK-2 and TRAAK in the human bladder revealed that TREK-1 is the predominantly expressed member of the mechano-gated subfamily of K2P channels. Immunohistochemical labelling of bladder wall identified higher levels of expression of TREK-1 in detrusor smooth muscle cells in comparison to bladder mucosa. Functional characterization and biophysical properties of the predominantly expressed member of the K2P family, the TREK-1 channel, were evaluated by in vitro organ bath studies and the patch-clamp technique. Electrophysiological recordings from single smooth muscle cells confirmed direct activation of TREK-1 channels by mechanical stretch and negative pressure applied to the cell membrane. Inhibition of TREK-1 channels in the human detrusor significantly delayed relaxation of the stretched bladder smooth muscle strips and triggered small-amplitude spontaneous contractions. Application of negative pressure to cell-attached patches (–20 mmHg) caused a 19-fold increase in the open probability (NPo) of human TREK-1 channels. l-Methionine (1 mm), a specific TREK-1 inhibitor, dramatically decreased the NPo of TREK-1 channels from 0.045 ± 0.003 to 0.008 ± 0.001 (n = 8, P ≤ 0.01). Subsequent addition of arachidonic acid (10 μm), a channel opener, increased the open probability of methionine-inhibited unitary currents up to 0.43 ± 0.05 at 0 mV (n = 9, P ≤ 0.05). The results of our study provide direct evidence that the response of the human detrusor to mechanical stretch is regulated by activation of mechano-gated TREK-1 channels. Impaired mechanosensation and mechanotransduction associated with the changes in stretch-activated K2P channels may underlie myogenic bladder dysfunction in humans.
Key points
Mechano-gated two-pore-domain potassium (K2P) channels are expressed in the human bladder, with TREK-1 being the predominant functional subunit.
TREK-1 channels in bladder smooth muscle are activated by membrane stretch and negative pressure applied to the patch pipette.
Inhibition of TREK-1 channels in the human detrusor significantly delays relaxation of bladder smooth muscle and triggers small-amplitude spontaneous contractions in response to stretch.
Application of negative pressure to cell-attached patches (–20 mmHg) causes a 19-fold increase in the open probability (NPo) of human TREK-1 channels.
l-Methionine (1 mm) dramatically decreases the NPo of TREK-1 channels from 0.045 ± 0.003 to 0.008 ± 0.001 (n = 8, P ≤ 0.01). Addition of arachidonic acid (10 μm) increases the open probability of methionine-inhibited unitary currents up to 0.43 ± 0.05 at 0 mV (n = 9, P ≤ 0.05).
TREK-1 channels may serve as a promising pharmacological target for bladder dysfunction in humans.
Introduction
The urinary bladder undergoes cyclic relaxation (filling phase) and contraction (voiding phase) during daily physiological activity. During bladder filling, there is usually no parasympathetic outflow from the spinal cord (de Groat, 2006); however, the bladder develops tone and also exhibits non-synchronized local contractions and relaxations that are caused by basal myogenic activity (Turner & Brading, 1997). Myogenic activity of the detrusor is defined as the ability of smooth muscle cells to generate mechanical activity independent of external stimuli (Andersson & Arner, 2004). Activation of mechanosensitive receptors on urothelial and smooth muscle cells is the first step in initiating mechanotransduction in the urinary bladder (Araki 2008; Andersson, 2010). Mechanical microforces during bladder filling are transmitted from smooth muscle cells to afferent fibres in the bladder wall, which propagate excitatory impulses to primary sensory neurons and, further, to the CNS, causing sensations of urgency when the bladder becomes full (Brading, 2006; Araki et al. 2008).
The membrane of detrusor smooth muscle cells presents a major target for the external mechanical forces that act upon a cell, and mechano-gated ion channels play a crucial role in the cellular physiology of mechanotransduction. They detect and transduce external mechanical forces into electrical and chemical intracellular signals (Martinac, 2004). Animal data suggest that stretch-activated two-pore-domain (K2P, KCNK) K+ channels play a critical role in bladder mechanosensitivity (Baker et al. 2008, 2010). They belong to a large family of mammalian K2P channels, which includes six functional classes (Kim, 2005). The structure of K2P channels consists of four transmembrane segments, two pore domains and an extended M1P1 extracellular loop with intracellular N- and C-termini. Despite this structural similarity, members of the K2P family share low sequence identity outside the pore regions (Patel & Honoré, 2001; Patel et al. 2001).
The TREK-1 (KCNK2, K2P2.1), TREK-2 (KCNK2, K2P10.1) and TRAAK (KCNK4, K2P4.1) channels are members of the TREK subfamily of mechanosensitive K2P channels. They are activated by physical and chemical stimuli such as membrane stretch, pH and lipids (Maingret et al. 1999a,b, 2000a,b; Bang et al. 2000; Kang et al. 2005). These channels regulate smooth muscle excitability by controlling the resting membrane potential (Koh & Sanders, 2001; Patel & Honoré, 2001; Sanders & Koh, 2006). Mechano-gated K2P channels are highly expressed in the smooth muscle of visceral hollow organs that undergo significant physiological stretch (Wellner & Isenberg, 1994; Koh et al. 2001; Bai et al. 2005; Tichenor et al. 2005; Baker et al. 2010), where they participate in a variety of important physiological functions (Franks & Honoré, 2004; Hamill, 2006; Honoré, 2007). In humans, the members of the mechanosensitive K2P channel family are also abundantly expressed in the nervous system, including brain regions (prefrontal cortex, hippocampus, hypothalamus and midbrain) and sensory neurons of the dorsal root ganglia (Hervieu et al. 2001; Medhurst et al. 2001; Heurteaux et al. 2006).
Although the presence of mechano-gated K2P channels in smooth muscle cells of non-human (Koh et al. 2001; Baker et al. 2008, 2010) and human urinary bladder (Bai et al. 2005; Baker et al. 2008; Buxton et al. 2010) has been documented previously, the expression profiles of different family members, their distribution in the bladder wall and their physiological role in the human detrusor have not been investigated. Moreover, little information is available regarding electrophysiological characteristics of single channels of TREK-1, a predominantly expressed member of the mechano-gated K2P family, in native bladder smooth muscle (BSM) cells.
Defining the biophysical properties of mechano-gated K2P channels and understanding their physiological role in native human tissue are of high translational value. This knowledge is essential for the validation of animal models reflecting human bladder function in pathophysiological conditions as well as for the development of novel therapeutic approaches for the treatment of myogenic bladder dysfunction in patients. The objective of this study was to examine the expression of stretch-activated K2P potassium channels (TREK-1, TREK-2 and TRAAK) in human BSM and reveal their functional role in BSM excitability and contractility.
Methods
Subject recruitment and collection of urinary bladder specimens
All experiments were conducted on human urinary bladder specimens obtained during cystectomies from patients diagnosed with bladder cancer. The protocol for the use of a healthy part of the urinary bladder from surgical waste tissue was approved by the University of Pennsylvania Institutional Review Board (#812269) and allowed the collection of specimens from 15 adult subjects (10 men and five women, 41–78 years old). The inclusion criteria for the study were as follows: (i) no symptoms of detrusor overactivity (American Urological Association Symptom Score <8); and (ii) negative dipstick or urinalysis prior to surgery. Specimens were not obtained from patients meeting the following exclusion criteria: use of narcotic analgesics for more than 3 days per week; impaired liver or kidney function (creatinine ≥2 mg dl−1); presence of discrete anatomical urinary tract abnormalities (e.g. urethral strictures, bladder diverticula or fistula) as determined by cystoscopy; current or recent (≤3 months) medications for overactive bladder; sacral neurostimulation; presence of a known neurological disorder (multiple sclerosis, Parkinson's disease, spina bifida or spinal cord injury/trauma); diabetes mellitus; a history of previous malignancy and/or treatment with chemotherapy/radiotherapy; women with current or prior (within previous 6 months) pregnancy or breast feeding.
The bladder specimens were delivered immediately upon availability from the operating room to the Pathology Laboratory at the Hospital of the University of Pennsylvania (located in the same building), followed by complete evaluation of the whole bladder by an attending pathologist. Healthy full-thickness bladder tissue (∼4.5–6.5 cm2) was excised from the bladder dome not closer than 1–1.5 cm from the edge of the malignant spot, placed in ice-cold Ca2+-free Tyrode solution (Table 1) and delivered to the laboratory. The advantages of this experimental approach included the opportunity of obtaining a relatively large piece of full-thickness human bladder within a short period of time after surgical removal, allowing preservation of the internal milieu of the human specimens and minimization of the modulating effects of in vitro conditions. Each full-thickness bladder specimen was divided under the dissecting microscope into several pieces. One full-thickness section of the tissue was placed in 4% paraformaldehyde solution for fixation followed by histological analysis and immunohistochemical (IHC) labelling. The rest of the tissue was dissected under the microscope to separate the mucosa from the detrusor. Small pieces of the mucosa and detrusor were frozen for RNA and protein analysis; the rest of the fresh detrusor tissue was used for in vitro contractility studies and isolation of single cells required for electrophysiological experiments and single-cell IHC.
Table 1.
Composition of solutions for in vitro studies
| Bath solutions (mm) | ||||
|---|---|---|---|---|
| Whole-cell mode | Cell-attached and inside-out mode | |||
| Tyrode solution | Normal K+ | Inside-out mode High K+ | Pipette solution (mm) | |
| NaCl, 137 | NaCl, 135 | NaCl, 135 | KCl, 40 | Potassium aspartate, 100 |
| KCl, 2.7 | KCl, 5.4 | KCl, 5.4 | Hepes, 10 | KCl, 30 |
| CaCl2, 10 μm | NaH2PO4, 0.33 | NaH2PO4, 0.33 | MgCl2, 1 | Hepes, 5 |
| MgCl2, 1 | MgCl2, 1 | MgCl2, 1 | CaCl2, 1 | MgCl2, 2 |
| NaH2PO4, 0.36 | CaCl2, 1 | CaCl2, 1 | — | EGTA, 1 |
| NaHCO3, 12 | Hepes, 5 | Hepes, 5 | — | — |
| C6H12O6, 5.5 | C6H12O6, 5.5 | C6H12O6, 5.5 | — | — |
| pH adjusted to 7.4 with NaOH | pH adjusted to 7.4 with NaOH | pH adjusted to 7.4 with NaOH | pH adjusted to 7.4 with KOH | pH adjusted to 7.2 with KOH |
Isolation of single smooth muscle cells from the human detrusor
Single human BSM cells were isolated from smooth muscle strips as previously described (Jin et al. 2004). Briefly, a small piece of the denuded BSM tissue was cut into 2–3 mm strips and placed in low-Ca2+ Tyrode solution (Table 1) supplemented with collagenase (1.3 mg ml−1; Yakult, Tokyo, Japan), bovine serum albumin (BSA; 2 mg ml−1; Sigma-Aldrich, St Louis, MO, USA) and trypsin (2 mg ml−1; Sigma-Aldrich). The tissue was placed in a 37°C water-bath for 10–12 min followed by trituration through a blunt-tipped glass pipette. Next, the tissue was transferred to a second tube with low-Ca2+ Tyrode solution containing BSA (2 mg ml−1) and collagenase (1 mg ml−1) for further incubation for 15–20 min at 37°C. After incubation, the BSM tissue was washed and transferred to fresh solution containing BSA only. Individual BSM cells were released by trituration through a series of blunt pipettes with decreasing tip diameters. A few drops of the solution containing dispersed BSM cells were placed on poly-l-ornithine-coated 35 mm coverslips with a glass window, allowed to settle down for 15–25 min and then were either used for electrophysiological recordings or fixed with 4% paraformaldehyde followed by immunocytochemical labelling. Live non-contracted human BSM cells were characterized by a spindle-like shape and a bright edge under the light microscope.
Tissue and single-cell immunolabelling
Section slices (8 μm) of full-thickness bladder specimens were made from paraffin blocks. Tissue sections were put into xylene to remove paraffin and descending concentrations of alcohol (100, 95, 70 and 30% in 1× PBS) before antigen retrieval treatment (10 mm sodium citrate, pH 6.0, incubated at 95°C for 10 min). The sections were kept in blocking solution (3% BSA) for 30 min. After blocking, the sections were incubated with the primary antibody, rabbit polyclonal anti-TREK-1 (sc-50412; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), at 1:400 dilution overnight at 4°C. A negative control to confirm the specificity of immunolabelling was prepared using non-immune rabbit serum in place of the primary antibody. Sections were washed three times in PBS and treated with secondary anti-rabbit Alexa Fluor 488 antibody (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:400 for 1 h. After additional washing, the sections were mounted on slides with a drop of mounting medium (Vector Laboratories Inc., Burlingame, CA, USA).
For staining single BSM cells, a few drops of freshly made cell suspension were placed on a coverslip with a glass window, and cells were allowed to settle down for 30 min at 37°C in an incubator. The solution was removed, and warm 4% paraformaldehyde was added to the coverslip for 10 min. Cells were then washed twice in PBS, blocked, and permeabilized in PBS with 10% normal donkey serum and 0.1% Triton X-100 for 30 min. After washing with PBS, cells were incubated with rabbit polyclonal primary anti-TREK-1 antibodies (sc-50412; Santa Cruz) at 1:400 dilution at 37°C for 2 h followed by labelling with secondary Cy3-conjugated anti-rabbit IgG antibodies, at 1:200 dilution (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h in the dark. After labelling, cells were washed with PBS and incubated with rabbit polyclonal anti-α-smooth muscle actin antibody at 1:200 dilution (ab5694; Abcam, Cambridge, MA, USA) and secondary antibody, Cy5-conjugated anti-rabbit IgG at 1:200 dilution (Jackson ImmunoResearch, West Grove, PA, USA), for 1 h in the dark. A separate coverslip was processed for single-cell IHC with the fluorescent antibody phalloidin-Atto 488, which labels F-actin (P5282; Sigma-Aldrich). A number of controls were employed for IHC studies, as follows: (i) the use of an unrelated antibody or PBS in place of the primary antibody; (ii) the use of the secondary antibody or the fluorochrome-conjugated antibody alone; and (iii) the use of PBS in place of the secondary antibodies. In all instances, control preparations showed either no labelling or background labelling. Slides were viewed under a confocal laser scanning biological microscope (Olympus, Center Valley, PA, USA). Images were captured and analysed using Fluoview FV1000 software (Olympus).
Isolation of RNA and quantitative RT-PCR
Total RNA was extracted from full-thickness bladder specimens with TRIzol reagent following the protocol from Invitrogen as previously described (Pan et al. 2010; Lei et al. 2013). First-strand cDNA was synthesized from 5 μg of the total RNA with 200 U of the SuperScript III reverse transcriptase (catalogue no. 18080-051; Invitrogen) in the presence of 40 U RNaseOUT, 10 mm DTT, dNTP mix at 10 mm and 50 μm of Oligo (dT)20. Real-time RT-PCR was run on a 7500 Fast Real Time PCR system (Applied Biosystems, Foster City, CA, USA) with 2 μl of cDNA for each reaction. TaqMan Gene Expression Master Mix (4369016-PEC) and TaqMan™ primer/probe kits were used for each specimen run in triplicate. The following TaqMan Gene Expression Assays (Applied Biosystems) were used in this study: human TREK-1 (KCNK2, Hs01005159-m1, amplicon size 109 bp), TREK-2 (KCNK10, Hs00368341-m1, amplicon size 78 bp), TRAAK (KCNK4, Hs00213267-m1, amplicon size 101 bp) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase, Hs02758991-g1, amplicon size 93 bp). The triplicate quantitative RT-PCR products from the same specimen were combined and subsequently run on 2% agarose gel followed by the analysis of the images using the Doc-It LS Image Acquisition & Analysis System (UVP, LLC, Upland, CA, USA).
Western blotting
Total protein was isolated from denuded BSM strips as previously described (Malykhina et al. 2013a,b). Briefly, tissues were homogenized in ice-cold lysis buffer containing 25% glycerol and 62.5 mm Tris–HCl, supplemented with protease inhibitor cocktail tablets and phosphatase inhibitor cocktail tablets. Ten per cent SDS was added to the samples, which were subsequently vortexed and boiled for 4 min. The extracts were centrifuged at 16,400×g for 15 min at 4°C, and supernatants with the total protein were collected. The protein concentration in each sample was detected using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). The amount of protein loaded per lane was 25 μg. Proteins were separated by 4–12% SDS-PAGE, transferred to nitrocellulose membrane and blocked with ODYSSEY blocking buffer for 45 min at room temperature. The membranes were incubated with the primary antibodies rabbit polyclonal anti-TREK-1 (sc-50412; Santa Cruz) at 1:200 dilution or goat polyclonal anti-TREK-1 (sc-11557; Santa Cruz; used for immunoprecipitation), goat anti-TREK-2 (sc-11560; Santa Cruz) at 1:200 dilution, goat anti-TRAAK (sc-11324; Santa Cruz) at 1:200 dilution, mouse anti-α-actin (sc-17829; Santa Cruz) at 1:800 dilution or mouse anti-GAPDH (sc-32233; Santa Cruz) at 1:800 dilution at 4°C overnight. Each antiserum was pre-incubated with the appropriate immunizing peptide as a control experiment to block reactivity. After washing, the membranes were incubated with secondary antibodies ECL Plex goat-anti-rabbit-IgG-Cy5 (PA45011; Lumigen, GE Healthcare, Southfield, Michigan, USA) at 1:2500 dilution or ECL Plex goat-anti-mouse IgG-Cy3 (PA43009; Lumigen, GE Healthcare) at 1:2500 dilution for 1 h at room temperature, followed by scanning on a Fujifilm Image Reader LAS-3000 (Fujifilm, Valhalla, NY, USA). The amount of protein in a band was determined by optical density scanning using Multi Gauge V 3.0 software (Fujifilm).
Immunoprecipitation protocol
Total protein lysates isolated from human bladder specimens were diluted with RIPA buffer without SDS [50 mm Tris–HCl (pH 7.5), 150 mm NaCl, 1% NP40, 0.5% sodium deoxycholate, 5 mm EDTA, protease inhibitor cocktail tablet (catalogue no. 11 836 170 001; Roche Applied Science (Indianapolis, IN, USA); one tablet per 10 ml of lysis buffer) and phosphatase inhibitor cocktail tablet (catalogue no. 04 906 837 001; Roche Applied Science; one tablet per 10 ml of lysis buffer)]. Protein samples underwent an immunoprecipitation protocol using a Pierce Crosslink Immunoprecipitation Kit (Pierce Biotechnology, Rockford, IL, USA). Ten micrograms of anti-TREK-1 rabbit polyclonal antibody (sc-50412; Santa Cruz) and control rabbit IgG were used for the immunoprecipitation–Crosslink assay. The protocol included the binding of antibody to protein A/G resin followed by crosslinking the bound antibody. The final protein samples were then subjected to Western blotting using goat polyclonal anti-TREK-1 antibody (sc-11557; Santa Cruz; 1:200 dilution) followed by incubation with secondary antibodies and analysis of the gels as described in the previous subsection for the Western blotting procedure.
Contractility studies
Bladder smooth muscle strips (∼50 mg each, 2–3 mm wide, 7–8 mm long) were dissected out of detrusor muscle specimens without mucosa and positioned in individual organ baths containing 10 ml of Tyrode buffer (37°C) equilibrated with 95% O2–5% CO2. One end of each strip was attached to a glass rod at the bottom of the organ chamber (15 ml; Radnoti LLC, Monrovia, CA, USA), while the other end was attached to a force displacement transducer connected to a computerized system for data acquisition and analysis (AD Instruments, Colorado Springs, CO, USA). After a 1 h equilibration, the length of optimal force development (Lo) was determined by manually increasing the length of each strip by 1.5 mm increments until the maximal contractile force in response to electric field stimulation (EFS; 80 V, 1 ms and 32 Hz) was achieved. The bath solution was then changed to fresh Tyrode buffer containing 1 μm of TTX (Sigma-Aldrich) to reduce the potential confounding effects of nerve stimulation on BSM contractility. The strips were incubated for 30 min, followed by the treatments with either arachidonic acid (AA; 10 μm) or l-methionine (1 mm) for 40 min. Untreated muscle strips served as controls. At the end of the incubation period, all strips were stretched by 30% of the initially established Lo, which allowed for complete relaxation in order to assess the effects of pharmacological modulation of TREK-1 channels on BSM relaxation. Following the stretch protocol, a KCl test (bath solution was replaced with Tyrode solution containing 125 mm KCl) was performed to evaluate phasic contractility of the BSM to ensure that the BSM could maintain the force after stretch.
Electrophysiological recordings
A few drops of the solution containing single freshly isolated BSM cells were placed in an experimental chamber of the patch-clamp set-up. Cells were allowed to adhere to the glass bottom of the chamber (20–30 min). Live BSM cells had an elongated shape and bright edges under a light microscope. Cells were used for patch-clamp recordings within 12 h after isolation. An Axopatch 200B amplifier, Digidata 1440 A and pCLAMP 10.2 software (Axon Instruments, Foster City, CA, USA) were used for electrophysiological recordings of TREK-1 currents. The glass pipettes for patch-clamp experiments were made from borosilicate glass (Sutter Instruments, Novato, CA, USA) and pulled using a Flaming-Brown horizontal puller (model P-97; Sutter Instruments). The pipettes were polished with a Micro Forge MF-830 pipette polisher (Narishige Group, Tokyo, Japan). Whole-cell, cell-attached and inside-out patch-clamp configurations were used in this study. In order to isolate TREK-1 currents, the bath solution contained TEA (5 mm) to block voltage-gated K+ channels, 4-aminopyridine (4-AP; 1 mm) to block outwardly rectifying K+ channels and nifedipine (1 μm) to block voltage-gated Ca2+ channels. All whole-cell and single-channel recordings were performed with charybdotoxin (200 nm) in the pipette to block Ca2+-activated K+ (BK) channels. The resistance of the pipettes was ∼5 MΩ for the whole-cell configuration and 8–10 MΩ for cell-attached or inside-out single-channel recordings when filled with pipette solution. Negative pressure was applied to patches using an ALA High Speed Pressure Clamp (HSPC-1; ALA Scientific Instruments, Westbury, NY, USA). In all experiments with the Pressure Clamp device, a gigaohm seal was established by applying 4–6 mmHg of negative pressure to the patch pipette. The pressure was controlled by a command voltage waveform input generated by Clampex software. Data were low-pass filtered at 5 kHz and sampled at a rate of 10–100 μs per sample. All patch-clamp experiments were carried out at room temperature.
Solutions and drugs
All chemicals and drugs were obtained from Sigma-Aldrich unless mentioned otherwise. l-Methionine was purchased from Fluka Biochemika (Buchs, Switzerland) and latrunculin A from Cayman Chemical (Ann Arbor, MI, USA). Solutions used for BSM cell isolation, contractility studies and electrophysiological recordings are listed in Table 1. The low-Ca2+ Tyrode solution was equilibrated with 95% O2–5% CO2. The bath solution was constantly bubbled with 100% O2 during electrophysiological recordings. In all patch-clamp experiments, extracellular solution contained TEA (5 mm) to block voltage-gated K+ channels, 4-AP (1 mm) to block outward rectifiers and nifedipine (1 μm) to block voltage-gated Ca2+ channels. All whole-cell and single-channel recordings were performed with charybdotoxin (200 nm) in the pipette to block Ca2+-activated K+ (BK) channels.
Data analysis and statistics
Contractility and relaxation of BSM in organ bath studies were recorded and analysed using a computerized system for data acquisition and analysis (AD Instruments). The following parameters were measured and analysed: the peak force (PF; in grams), integral force (IF) and the slope (S; in grams per second) of BSM contraction (Sc) and relaxation (Sr). The peak force (PF; in grams) is the maximal amplitude of contraction in response to respective stimulation; the integral force (IF) is the area under the curve of a single contraction after application of KCl or stretch; and the slope (S; in grams per second) reflects the maximal velocity of development of the contractile (Sc) or relaxant response (Sr), suggestive of how fast the muscle contracts/relaxes in response to stimulation. These parameters are shown in the inset to Fig. 4D.
Figure 4. Role of TREK-1 in relaxation of isolated BSM strips upon stretch and pharmacological testing.
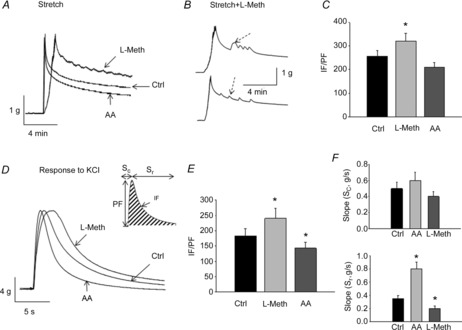
A, representative recordings from BSM strips upon stretch after incubation with either arachidonic acid (AA) or l-methionine (L-Meth; N = 3, n = 8). Abbreviation: Ctrl, control. B, examples of stretch recordings showing small-amplitude contractions (indicated by arrows) during the relaxation phase in strips incubated with l-methionine (N = 3, n = 7). C, analysis of integral force (IF) normalized to the peak force (PF) in strips exposed to TREK-1 modulators and stretch. D, contractility and relaxation of BSM in response to a KCl test. Inset shows a scheme of the measured parameters that were used for statistical analyses. E, normalized values of IF/IP of muscle response to KCl after pharmacological modulation of TREK-1 channels. F, top graph summarizes the maximal slope of the contractile phase of KCl response (Sc) after AA and l-methionine applications; bottom graph includes the slope values for muscle relaxation (Sr).
Electrophysiological recordings were performed using whole-cell, cell-attached and inside-out patch-clamp configurations. During whole-cell recordings, the cells were held at −50 mV between the voltage steps applied from −80 to +60 mV (500 ms, 10 mV increments). The amplitude of the outward current was measured at the peak value for each voltage and normalized to the cell size for the current–voltage plot. A voltage-ramp protocol was run from −100 mV to +100 mV (10 traces with 5 s intervals) to evaluate the voltage threshold for TREK-1 channel activation. Single-channel currents were analysed by using Fetchan and pStat subroutines in the pClamp 10.2 software (Molecular Devices, Sunnyvale, CA, USA) and SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA, USA). For analysis of the single-channel kinetics, the amplitude of the TREK-1 current and distribution of open/closed times were measured by constructing histograms after events-list analysis from raw records followed by Gaussian distribution fit. Events were detected by setting a threshold at one-half of the amplitude of the open-channel current. Channel activity was calculated as the channel open probability (NPo). The NPo was measured by using the built-in algorithm in Clampfit 10.2, which calculates NPo as To/(To + Tc), where To and Tc are the total open and closed times, respectively, for number of channels (N) in the patch. Single-channel currents were analysed within 1–3 min intervals prior to and after the pharmacological testing of single-channel events. The graphs for mean whole-cell and single-channel current–voltage as well as NPo–voltage dependence were made using SigmaPlot 11.0 software (Systat Software Inc.).
In all experiments, N reflects the number of human specimens, while n is the number of analysed recordings/sections/strips. Statistical analyses were performed using n values and are presented as means ± SEM. Statistical significance between the groups was assessed using SigmaPlot 11.0 software (Systat Software Inc.) by two-way ANOVA followed by Bonferroni's test when appropriate. A difference between the groups with P ≤ 0.05 was considered statistically significant.
Results
Gene and protein expression of mechano-gated K2P channels in the human bladder
Real-time RT-PCR was performed on full-thickness bladder specimens (N = 6) to evaluate the presence of mRNA transcripts for K2P channels. Amplicons for TREK-1 were detected in all tested samples (Fig. 1A), whereas the amplicons for TREK-2 were identified in two of six specimens. TRAAK mRNA was found in two of six samples which did not express TREK-2. Analysis of relative mRNA expression normalized to the GAPDH level in each specimen revealed 6-fold higher transcript levels for TREK-1 (0.59 ± 0.07; Fig. 1B) compared with TREK-2 (0.09 ± 0.02, P ≤ 0.05) and TRAAK (0.02 ± 0.01, P ≤ 0.05).
Figure 1. Gene and protein expression of mechanosensitive K2P channels in the human bladder.
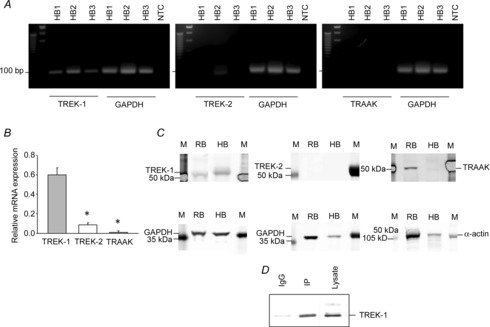
A, representative gel images of quantitative RT-PCR products of mechanosensitive K2P channels TREK-1 (Kcnk2), TREK-2 (Kcnk10) and TRAAK (Kcnk4) from three full-thickness urinary bladder specimens (HB1, HB2 and HB3). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Abbreviation: NTC, no template control. The expected amplicon sizes were 109 bp (TREK-1), 78 bp (TREK-2), 101 bp (TRAAK) and 93 bp (GAPDH). B, mRNA levels of K2P channels normalized to the respective GAPDH mRNA level revealed higher gene expression of TREK-1 than TREK-2 or TRAAK in human bladder specimens. C, representative Western blots displaying protein expression of mechanosensitive K2P channels in the human detrusor (denuded). Protein was combined from the three human specimens shown in A. Human samples were run in parallel with rat bladder samples for comparison. Abbreviations: HB, human bladder (detrusor); M, marker; and RB, rat bladder (detrusor). D, immunoprecipitation (IP) protocol revealed a single band for TREK-1.
We next examined the protein expression of TREK-1, TREK-2 and TRAAK in the denuded detrusor smooth muscle. Figure 1C shows the protein expression of K2P channels in the human BSM. The human samples were run in parallel with protein isolated from the rat detrusor for comparison. The size of the TREK-1 band in the human bladder was ∼50–52 kDa. TREK-2 and TRAAK were undetectable at the protein level. To confirm that we isolated TREK-1 protein from the human detrusor, we immunoprecipitated TREK-1 from the total lysate as described in the Methods, followed by Western blotting analysis. Figure 1D shows a single band for TREK-1 after the immunoprecipitation protocol. These results provide evidence that TREK-1 is the main mechano-gated K2P channel expressed in the human bladder smooth muscle.
Immunohistochemical localization of TREK-1 in the bladder wall
Full-thickness bladder specimens (N = 10) were processed for immunohistochemical labelling to determine the morphological distribution of the TREK-1 channel in the bladder wall. After labelling paraffin sections (8 μm) with anti-TREK-1, examination of cross-sections by confocal microscopy showed weak and uneven immunoreactivity throughout the bladder mucosa (Fig. 2A). Examination at higher magnification (Fig. 2B) revealed increased intensity of labelling in fibre-like structures located underneath the urothelium, which may represent the nerve terminals supplying the bladder mucosa. No immunoreactivity was observed in the negative control specimen, where TREK-1 antibody had been pre-absorbed with its control antigen (data not shown). Evaluation of smooth muscle bundles in the human bladder confirmed higher expression of TREK-1 channels in the detrusor in comparison with mucosa (Fig. 2C and D).
Figure 2. Immunohistochemical labelling of TREK-1 channel in human urinary bladder.
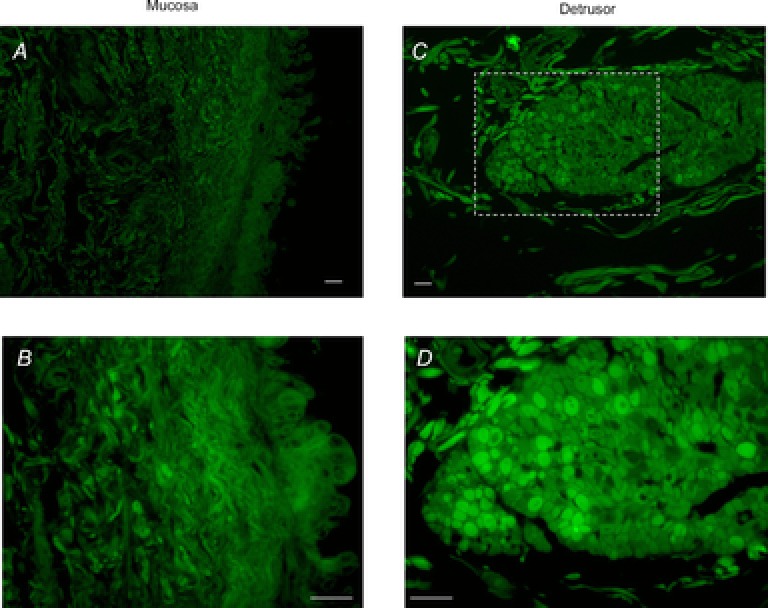
A, laser-scanned confocal micrograph represents TREK-1 immunolabelling of the bladder mucosa. B, higher magnification shows a weaker signal in the urothelium in comparison to fibre-like structures located in the lamina propria. C, expression of TREK-1 in a single smooth muscle bundle. D, increased magnification of the boxed area selected in C confirms a higher level of TREK-1 expression in the detrusor muscle when compared with the bladder mucosa (N = 10). No immunoreactivity was observed in the negative control, in which TREK-1 antibody was pre-absorbed with its respective control antigen (not shown). Scale bars: A and C, 0.5 mm; B and D, 250 μm.
Expression of the TREK-1 channel on the membrane of BSM cells was confirmed at the single-cell level. Figure 3A shows the labelling of freshly isolated detrusor myocytes with phalloidin, which binds to polymerized F-actin. This labelling allowed visualization of the original spindle-like shape of native BSM cells in the relaxed (non-contracted) state. Double labelling of detrusor myocytes with TREK-1 and smooth muscle α-actin antibodies revealed predominant membrane expression of TREK-1, whereas α-actin was distributed throughout the cell (Fig. 3B–D).
Figure 3. TREK-1 labelling in single freshly dispersed human bladder smooth muscle (BSM) cells.
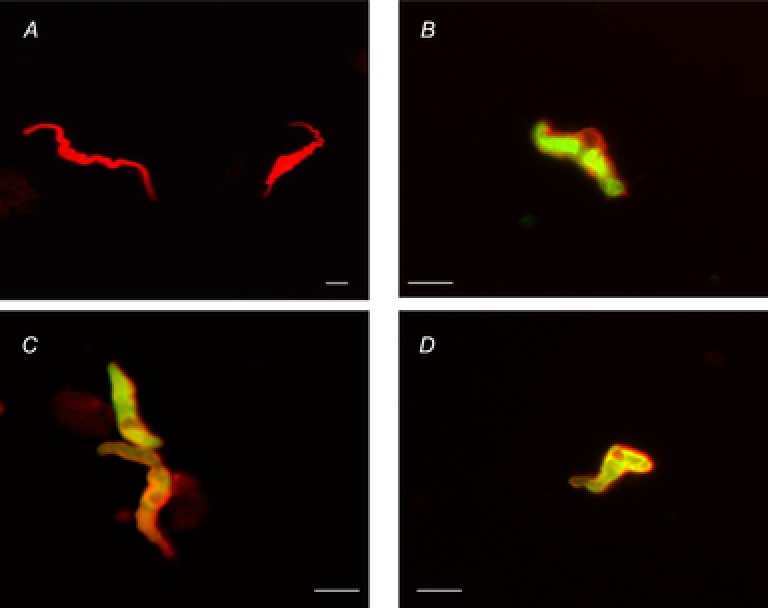
A, phalloidin (F-actin) labelling of the freshly dispersed human BSM cells. Note the unique cellular morphology (spindle-like shape) of the relaxed smooth muscle cells. Scale bar represents 25 μm. B, C and D show the labelling of smooth muscle cells with TREK-1 (red) and smooth muscle α-actin (green) isolated from three different BSM specimens. Scale bars represent 50 μm.
Contractility and relaxation of human BSM strips upon stretch and pharmacological modulation of TREK-1 channels
Isolated BSM strips (average weight 58 ± 12 mg) were incubated with TREK-1 activator and inhibitor after establishing the optimal length of muscle contraction (Lo) as previously described (Hypolite et al. 2013). Untreated strips served as an internal control. After incubation with the drugs, each muscle strip was stretched by 30% over the established Lo, followed by full relaxation. Figure 4A shows the representative raw traces recorded from BSM strips upon stretch after exposure to either AA or l-methionine. Arachidonic acid, a TREK-1 opener, increased the rate of muscle relaxation; however, the difference did not reach statistical significance upon normalization of the results (Fig. 4C). Application of l-methionine, a TREK-1 inhibitor, caused a substantial delay in BSM relaxation after stretch (Fig. 4A), with many strips having spontaneous small-amplitude contractions during the relaxation phase. Examples of these spontaneous contractions are included in Fig. 4B. Analysis of integral force normalized to the peak force in strips with l-methionine determined a 20% increase of the area under the stretch curve (IF/PF; Fig. 4C; n = 7, P ≤ 0.05 to control strips). A subsequent KCl test also revealed significant differences in BSM relaxation after pharmacological modulation of the TREK-1 channel. The IF/IP value was increased by 31% after l-methionine application (Fig. 4D and E; n = 7, P ≤ 0.05) suggestive of a significant deceleration of muscle relaxation, whereas the IF/IP value after AA exposure was reduced by 22% (Fig. 4D and E; n = 8, P ≤ 0.05), which confirms acceleration of muscle relaxation upon activation of TREK-1. Additional analysis of the maximal slope of the contractile (Sc) and relaxing phases (Sr) of BSM response to KCl established that AA increased the velocity of relaxation by 63% (Fig. 4F, bottom graph, Sr; n = 8, P ≤ 0.05), whereas l-methionine decreased Sr by 35% (Fig. 4F, bottom graph, Sr; n = 7, P ≤ 0.05 to control). None of the drugs significantly affected the maximal slope of the contractile phase of KCl response (Sc; Fig. 4F, top graph).
Activation of mechanosensitive K+ channels by stretch
Biophysical properties of functional mechanosensitive K+ channels in human BSM cells were characterized by using the patch-clamp technique in the whole-cell mode, as well as cell-attached and excised patches. The TREK-1 channels were shown to be activated by application of membrane stretch, cell elongation and negative pressure (Koh & Sanders, 2001). Therefore, we tested the effects of cell elongation on the activation of whole-cell current by gentle pull of a micromanipulator away from the cell. Representative traces obtained before and after stretch in the same cell are shown in Fig. 5A. Stretch of the cell membrane caused a 2-fold increase in the peak amplitude of outward K+ currents recorded in an asymmetrical K+ concentration (n = 6, N = 5, P ≤ 0.05 for positive voltages starting from −20 mV).
Figure 5. Effect of stretch on TREK-1 current expressed in native human BSM cells.
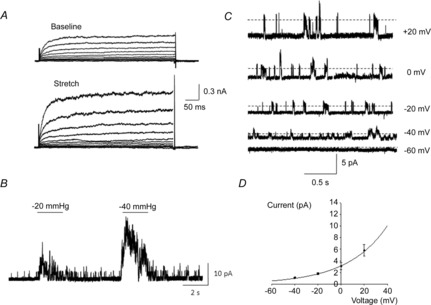
A, representative whole-cell currents recorded in bladder myocytes before (baseline) and after cell elongation using a micromanipulator (N = 5, n = 6). The membrane potential was stepped from −80 to +60 mV in 10 mV increments for 500 ms. B, bladder myocytes were exposed to negative pressure in the cell-attached mode at a holding potential of 0 mV in an asymmetrical K+ concentration (5/140 mm). Membrane stretch significantly increased the open probability of TREK-1 channels (N = 5, n = 6). C, representative recordings of single-channel activity in an asymmetrical K+ concentration (5/140 mm) at various holding potentials from inside-out excised patches. Dashed lines denote the channel open state. D, the current amplitude–voltage plot of the single-channel conductance fitted by the Goldman–Hodgkin–Katz equation in asymmetrical K+ solutions (N = 7, n = 15).
Activation of TREK-1 single-channel currents by stretch was recorded in cell-attached patches of BSM cells. The ALA High Speed Pressure Clamp was used to create a gigaohm seal by applying 4–6 mmHg of negative pressure to the patch pipette. Cells were not used for electrophysiological recordings if the pressure necessary to achieve a gigaohm seal exceeded −10 mmHg. Application of negative pressure to trigger activation of mechano-gated channels started 3–5 min after seal formation. Cell-attached patch-clamp recordings were performed at 0 mV in asymmetrical K+ concentrations (5/140 mm) to exclude contamination from non-selective cation channels. In order to isolate TREK-1 currents, the bath solution contained TEA (5 mm) to block voltage-gated K+ channels, 4-AP (1 mm) to block outward rectifying K+ channels and nifedipine (1 μm) to block voltage-gated Ca2+ channels. All whole-cell and single-channel recordings were performed with charybdotoxin (200 nm) in the pipette to block Ca2+-activated K+ (BK) channels. Membrane patches were exposed to negative pipette pressures from −20 to −40 mmHg (Fig. 5B). Application of negative pressure to cell-attached patches dramatically increased the open probability (NPo) of TREK-1 channels from 0.045 ± 0.003 to 0.83 ± 0.007 at −20 mmHg (n = 6 from 10 patches, N = 5, P ≤ 0.05 to baseline) and to 1.32 ± 0.01 at −40 mmHg (n = 7, N = 5, P ≤ 0.05). When atmospheric pressure was restored, the channel open probability returned to baseline levels. The TREK-1 channels were also strongly activated in inside-out patches due to patch excision.
We next measured the amplitude of unitary currents at different holding potentials (Fig. 5C). The channels activated in these conditions generated outward currents (i.e. K+ channels), and the amplitude of unitary currents was 3.1 ± 0.4 pA at 0 mV, which was similar to previously recorded values in BSM cells isolated from animal species (Koh et al. 2001; Koh & Sanders, 2001; Baker et al. 2008). The unitary current amplitude–voltage plot of the single-channel conductance was fitted with the Goldman–Hodgkin–Katz equation, suggesting that currents were due to K+ channels (Fig. 5D). In symmetrical K+ conditions (140/140 mm), the single-channel conductance was 97 ± 10 pS (n = 4, N = 3). These recordings demonstrate that BSM cells from the human detrusor express functional TREK-1 channels that are activated by membrane stretch.
Pharmacological modulation of TREK-1 currents in human BSM cells
Members of the mechano-gated K2P channel family are known to be activated by arachidonic acid. To test modulation of the recorded TREK-1 current by channel openers, we evaluated the effects of AA (10 μm) on the outward current in a whole-cell mode. Figure 6A shows representative traces of the TREK-1 current recorded from a BSM cell isolated from the human bladder. Application of AA caused a significant increase in the current amplitude, reaching 2-fold at +20 mV from 15.2 ± 6.7 to 35.8 ± 6.8 pA pF−1, and 4-fold at +50 mV from 27.7 ± 10.0 to 120.7 ± 10.8 pA pF−1 (Fig. 6B; n = 12, N = 8, P ≤ 0.05 to baseline current). The same increase in the current amplitude was noted after AA application during the ramp protocol when the voltage was gradually changed from −100 to +100 mV (Fig. 6C). The AA-induced current exhibited a reversal potential near −80 mV, indicating selective K+ conductance. The effects of AA were reversible and the current amplitude was significantly decreased after perfusion with the normal bath solution for 30 min (Fig. 6B and C).
Figure 6. Activation of TREK-1 currents by arachidonic acid (AA; channel opener) recorded in whole-cell voltage-clamp mode.
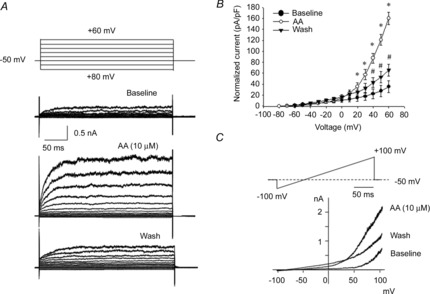
A shows representative TREK-1 currents before (baseline) and after application of AA. The membrane potential was stepped from −80 to +60 mV in 10 mV increments (500 ms) from the holding potential of −50 mV. B, current amplitude–voltage relationship of TREK-1 current was normalized to cell capacitance (N = 8, n = 12). *P ≤ 0.05 relative to baseline; #P ≤ 0.05 relative to AA-evoked current. C, effect of AA application on TREK-1 current recorded in response to a voltage-ramp protocol from −100 to +100 mV. Note that there is a significant increase in outward current after AA and a decrease after washing with normal solution.
The effects of AA on the open probability of mechanosensitive K+ channels were also tested in cell-attached and excised patches. As mentioned earlier, excision of a membrane patch significantly increased NPo of single channels. Application of AA to excised patches held at 0 mV in an asymmetrical K+ concentration (5/140 mm) caused a gradual increase in channel openings (Fig. 7A). Analysis of TREK-1 NPo in asymmetrical K+ solutions at different voltages did not reveal significant changes from negative to positive potentials (Fig. 7B), suggesting that activation of the channel is mainly voltage independent. However, treatment with AA increased NPo of TREK-1 channels in asymmetrical K+ solutions by 10-fold from 0.045 ± 0.003 to 0.42 ± 0.04 at 0 mV (n = 9, N = 6, P ≤ 0.05; Fig. 7C).
Figure 7. Pharmacological modulation of TREK-1 channel by AA at the single-channel level.
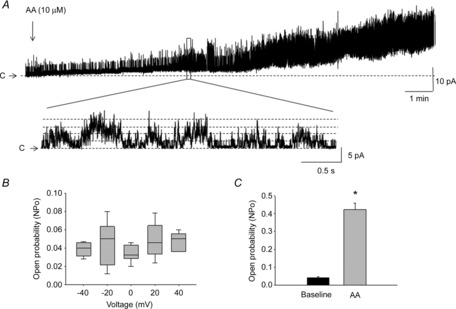
A, representative trace of TREK-1 current recorded upon application of AA (10 μm, upper panel) in an asymmetrical K+ concentration (5/140 mm) in an excised patch. Arachidonic acid significantly increased the open probability (NPo) of TREK-1 channels, as shown in the expanded portion of the trace below the main trace. ‘C’ denotes the channel closed level. B, TREK-1 single-channel open probability (NPo) at different voltages from −40 to +40 mV recorded in cell-attached mode in normal conditions. C, an increase in NPo of TREK-1 channels after application of AA in inside-out patches in an asymmetrical K+ concentration (5/140 mm, N = 6, n = 9). Asterisk in Fig. 7C means a statistically significant difference (p < 0.05 to baseline).
The blockade of TREK-1 channels by the amino acid l-methionine (1 mm) was evaluated in cell-attached patches at 0 mV in an asymmetrical K+ concentration (n = 8, N = 5; Fig. 8A). Application of l-methionine (1 mm, 5 min) dramatically decreased the open probability of TREK-1 channels (NPo = 0.008 ± 0.001, P ≤ 0.01; Fig. 8B, inset #1) compared to the baseline values. The effects of l-methionine were reversed by subsequent application of AA to the same patches. Addition of arachidonic acid (10 μm) increased the open probability of methionine-inhibited unitary currents to 0.43 ± 0.05 at 0 mV (n = 7, N = 5, P ≤ 0.05; Fig. 8B, inset #2). The amplitude histograms presented in Fig. 8 were obtained from 1 min of single-channel recordings in control conditions (Fig. 8C), during 5 min exposure to l-methionine (Fig. 8D) and after subsequent application of AA (Fig. 8E). The effects of both l-methionine and AA were reversible upon washout of the drugs. These data suggest that TREK-1 channels expressed in native human BSM cells are inhibited by the specific channel blocker l-methionine and activated by AA, a channel opener.
Figure 8. Effect of l-methionine on activation of TREK-1 channels.
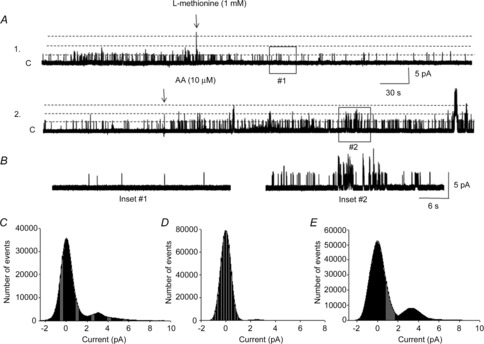
A, l-methionine (1 mm) decreased the open probability of TREK-1 channels and arachidonic acid (10 μm) increased the open probability of the channels recorded in cell-attached mode at 0 mV in an asymmetrical K+ concentrations. Trace #2 is a continuous extension of trace #1. ‘C’ denotes the channel closed level, and dashed lines denote channel openings. B shows portions of traces #1 and #2 on an extended time scale. The amplitude histograms were obtained from 1 min of recordings at the baseline (C), after l-methionine (D) and after AA (E) each fitted with a Gaussian distribution function to obtain the current amplitude value for each histogram (dotted bell-shaped curves).
Discussion
This study presents a comprehensive evaluation of the expression, morphological distribution and function of mechanosensitive K2P channels in the human bladder. Specifically, we found that TREK-1 is the predominantly expressed member of the mechano-gated subfamily of K2P channels in the detrusor smooth muscle. All tested bladder specimens expressed the transcript for the TREK-1 channel, followed by translation of fully functional protein, as confirmed by electrophysiological recordings. The results of organ bath and electrophysiological experiments confirmed the role of TREK-1 in human BSM excitability, contractility and relaxation upon stretch. TREK-1 single-channel openings stimulated by negative pressure applied to the patches showed no apparent voltage dependence for activation and had a conductance similar to the previously reported values (Koh et al. 2001; Baker et al. 2008). The open probability of the recorded channels was significantly increased by arachidonic acid and inhibited by l-methionine, reagents that activate and block TREK-1 channels, respectively (Patel et al. 1998; Franks & Honoré, 2004).
Mammalian K2P channels include six functional classes and belong to a large family of K+ channels, along with the inward rectifiers and the voltage- and/or calcium-dependent K+ channels. Each K2P channel subunit includes four transmembrane segments and two pore-forming (P) domains, which are arranged in tandem and function as either homo- or heterodimeric channels (Dedman et al. 2009). The presence of the mechano-gated subfamily of K2P channels in the human bladder has implications relative to bladder physiology. The urinary bladder experiences cyclic physiological expansions during the filling phase without significant changes in intravesical pressure or activation of BSM phasic contractions (Wellner & Isenberg, 1993; Ordway et al. 1995; Koh et al. 2001; Park et al. 2005; Baker et al. 2008; Day & Thompson, 2010). Members of the mechano-gated K2P channel family have also been identified in other visceral smooth muscle organs (e.g. gastrointestinal tract and uterus) that undergo significant physiological stretch (Ordway et al. 1995; Fink et al. 1996; Koh et al. 2001; Medhurst et al. 2001; Talley et al. 2001; Bai et al. 2005; Park et al. 2005; Tichenor et al. 2005; Baker et al. 2008; Buxton et al. 2010). In humans, TREK-1 and other subunits of this subfamily were detected in the human myometrium, where they contribute to the maintenance of uterine relaxation during gestation (Bai et al. 2005; Buxton et al. 2010). Further studies established that there are three wild-type human TREK-1 transcript isoforms in non-pregnant and pregnant human myometrium (Wu et al. 2012). In addition to visceral smooth muscle, human TREK-1 is expressed in the central and peripheral nervous system, including prefrontal cortex, hippocampus, hypothalamus, midbrain (dorsal raphe nucleus), cerebellum and sensory neurons of dorsal root ganglia (Medhurst et al. 2001; Heurteaux et al. 2006). Its physiological role in the nervous system is related to the control of electrogenesis, axonal migration and synaptogenesis (Fink et al. 1996; Heurteaux et al. 2004), as well as modulation of the neural response to temperature, mechanical stretch and volatile anaesthetics (Goldstein et al. 1998, 2001).
Immunohistochemical staining of the human bladder with anti-TREK-1 in our study revealed higher expression of the channel in the detrusor in comparison to the bladder mucosa. The pattern of the staining was ‘patchy’, reflecting variability in the expression level of TREK-1 protein among smooth muscle cells. Expression of ion channels usually undergoes cyclic fluctuations, with an average turnover time of 3 days. Our data correlate with the previous reports which compared the distribution of mRNA of K2P channels in the human cultured smooth muscle cells (Tertyshnikova et al. 2005). The mRNA profile revealed a detectable signal for TWIK-2 (K2P6.1), TASK-4 (K2P17.1) and TREK-1 (K2P2.1) channels in BSM cells. However, only TREK-1 had a 12-fold higher level of expression in BSM cells relative to the aortic smooth muscle (Tertyshnikova et al. 2005). Non-human animal studies previously showed differential expression of TREK-1 in the vascular system (Dedman et al. 2009). Immunohistochemical labelling of mouse mesenteric arteries revealed TREK-1 expression in both myocytes and endothelial cell layers (Garry et al. 2007). TREK-1 was also highly expressed in mouse cutaneous arteries, in the endothelium of the blood vessel network surrounding hair follicles (Garry et al. 2007), in rat and mouse basilar arteries but not in carotid or femoral arteries (Blondeau et al. 2007).
A particularly important functional property of TREK-1 is its mechanosensitivity, with channel opening induced by membrane stretch (Patel et al. 1998; Maingret et al. 1999a,b; Honoré et al. 2002). The concept of smooth muscle mechanosensitivity was first introduced in the 1950s, with the discovery that smooth muscle can respond to stretch in the absence of extrinsic neural input (Born & Bulbring, 1955; Bulbring, 1955). Later, the development of the patch-clamp technique led to the discovery of mechanosensitivity as an inherent property of some ion channels (Hamill, 2006), suggesting that mechano-gated ion channels transduce mechanosensitivity in a variety of cells. Indeed, cells containing mechanosensitive ion channels are able directly to sense and then respond to mechanical forces (Martinac, 2004; Sukharev & Anishkin, 2004; Sukharev & Corey, 2004) by altering the flow of ions across the cell membrane.
The mechanosensitivity of ion channels is modulated by the channel environment on the plasma membrane. Immunocytochemical co-labelling of TREK-1 with smooth muscle α-actin in freshly isolated human BSM cells determined that TREK-1 is located close to actin microfilaments. This correlates with previous studies that established co-localization of TREK-1 with phalloidin in COS7 cells transfected with TREK-1 (Lauritzen et al. 2005). Biophysical studies suggest that there is a dynamic interaction between TREK-1 and the actin cytoskeleton, with the cytoskeleton acting as a tonic repressor, limiting channel activation by membrane tension (Patel et al. 1998, 2001; Lauritzen et al. 2005).
Contractility experiments on isolated detrusor strips confirmed a key role of TREK-1 channels in relaxation of the stretched BSM. Inhibition of TREK-1 by l-methionine led to a significant deceleration of relaxation, with many strips exhibiting small-amplitude spontaneous contractions (shown in Fig. 4B). This effect could be explained partly by the ability of TREK-1 to alter the nature of the BSM syncytium. Previous animal studies established that inhibition of TREK-1 by methionine compounds allowed Ca2+ waves to spread over long distances within the detrusor, thereby increasing excitation within smooth muscle bundles (Baker et al. 2008). However, inhibition of TREK-1 channels did not significantly impact the ability of BSM to develop contractile force, as evidenced from the unaltered slope of the contractile phase of the muscle response to KCl. Our results, therefore, suggest that TREK-1 channels mainly participate in maintaining the relaxation of BSM upon physiological stretch without directly affecting the ability of the muscle to develop the contractile force necessary for the voiding phase.
Analysis of the electrophysiological properties of TREK-1 channels in human BSM cells confirmed direct activation of the channels by membrane stretch in the whole-cell mode and by negative pressure in cell-attached patches. The open probability of the recorded TREK-1 channels did not show apparent voltage dependence in the applied conditions. TREK-1 is unusual in terms of its pharmacology, because it is resistant to all of the classical blockers of voltage- and ligand-gated K+ channels (Fink et al. 1996; Patel et al. 1998). Previous animal studies on freshly isolated BSM cells established that application of 4-aminopyridine (5 mm), tetraethylammonium (1–10 mm) and changes in cytoplasmic Ca2+ concentration had no significant effect on the open probability of stretch-activated K2P channels (Monaghan et al. 2011). Our pharmacological testing evaluated the effects of AA, an activator of TREK-1 channels (Franks & Honoré, 2004), and l-methionine, a blocker of TREK-1 channels (Park et al. 2005; Baker et al. 2008), on the recorded currents. We determined that AA (10 μm) dramatically increased the open probability of TREK-1 channels, whereas l-methionine (1 mm) decreased the open probability of TREK-1 channels, as previously established for animal BSM (Koh et al. 2001; Baker et al. 2008, 2010) and human myometrium (Buxton et al. 2010). These results suggest that TREK-1 channels in the human bladder may be targeted pharmacologically in pathophysiological conditions.
Conclusions and clinical implications
Our data provide direct evidence that mechanosensitive K2P ion channels are important physiological regulators of human urinary bladder function, with TREK-1 being the predominantly expressed member of the mechano-gated K2P subfamily. Mechanosensitive K2P channels are activated by membrane stretch and, upon opening, cause hyperpolarization of the cell membrane, resulting in smooth muscle relaxation. This mechanism decreases bladder excitability and prevents voiding contractions, to enhance accommodation during progressive bladder filling.
Participation of K2P channels in the response of the human detrusor to physiological stretch suggests that TREK-1 channels may serve as a promising pharmacological target for bladder dysfunction. The contribution of TREK-1 channels to the development of pathological conditions causing detrusor under- or overactivity in humans is yet to be established. During the filling phase, detrusor smooth muscle demonstrates changes in tone and a number of subthreshold non-voiding contractions in patients with detrusor overactivity (Andersson, 2006, 2010). Pharmacological or genetic modulation of mechano-gated K2P channels with channel activators may reduce detrusor overactivity and overactive bladder in humans. Likewise, inhibition or suppression of these channels has the potential to increase BSM contractility and relieve detrusor underactivity, which is clinically associated with urinary retention. Further studies are warranted to evaluate the role of mechano-gated K2P channels in the human bladder in pathological conditions and develop appropriate selective therapeutics for the clinical setting.
Acknowledgments
We thank the residents and staff of the Division of Urology in the Department of Surgery, Department of Pathology, Collaborative Human Tissue network (CHTN) site at the University of Pennsylvania, and the National Research Disease Interchange (NDRI) for help with timely collection, processing and delivery of human bladder specimens; Mr Joseph Hypolite for in vitro contractility experiments; and Mr Matthew McMillan for assistance with preparation of the manuscript.
Glossary
- AA
arachidonic acid
- 4-AP
4-aminopyridine
- BSA
bovine serum albumin
- BSM
bladder smooth muscle
- IHC
immunohistochemical/immunohistochemistry
- K2P
two-pore-domain potassium channel
- NPo
channel open probability
- TEA
tetraethylammonium
Additional information
Competing interests
None declared. Preliminary results of this work were presented at the American Urological Association 2013 meeting in abstract form.
Author contributions
Q.L. and A.P.M. conceived and designed the research. Q.L., X.-Q.P., S.C. and A.P.M. performed the experiments, analysed the data and prepared the figures. Q.L., S.B.M., T.J.G. and A.P.M. interpreted the results of the experiments. A.P.M. drafted the manuscript. Q.L., X.-Q.P., S.C., S.B.M., T.J.G. and A.P.M. edited and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by the Harrison Department of Surgical Research, Department of Surgery, University of Pennsylvania and National Institutes of Health grant DK-077699 (to A.P.M.). We acknowledge the use of tissues procured by the National Disease Research Interchange (NDRI) with support from NIH grant 5U42 RR006042.
References
- Andersson KE. Treatment-resistant detrusor overactivity–underlying pharmacology and potential mechanisms. Int J Clin Pract Suppl. 2006;2:8–16. doi: 10.1111/j.1742-1241.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn. 2010;29:97–106. doi: 10.1002/nau.20784. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M. Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol. 2008;15:681–687. doi: 10.1111/j.1442-2042.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, Taggart MJ, Fyfe GK. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- Baker SA, Hatton WJ, Han J, Hennig GW, Britton FC, Koh SD. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol. 2010;183:793–800. doi: 10.1016/j.juro.2009.09.079. [DOI] [PubMed] [Google Scholar]
- Baker SA, Hennig GW, Han J, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K+ channels. Br J Pharmacol. 2008;153:1259–1271. doi: 10.1038/sj.bjp.0707690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res. 2007;101:176–184. doi: 10.1161/CIRCRESAHA.107.154443. [DOI] [PubMed] [Google Scholar]
- Born GV, Bulbring E. The effect of 2:4-dinitrophenol (DNP) on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1955;127:626–635. doi: 10.1113/jphysiol.1955.sp005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570:13–22. doi: 10.1113/jphysiol.2005.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955;127:9P. [PubMed] [Google Scholar]
- Buxton IL, Singer CA, Tichenor JN. Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLoS ONE. 2010;5:e12372. doi: 10.1371/journal.pone.0012372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day IN, Thompson RJ. UCHL1 (PGP 9.5): neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327–362. doi: 10.1016/j.pneurobio.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Dedman A, Sharif-Naeini R, Folgering JH, Duprat F, Patel A, Honoré E. The mechano-gated K2P channel TREK-1. Eur Biophys J. 2009;38:293–303. doi: 10.1007/s00249-008-0318-8. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Honoré E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Garry A, Fromy B, Blondeau N, Henrion D, Brau F, Gounon P, Guy N, Heurteaux C, Lazdunski M, Saumet JL. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Rep. 2007;8:354–359. doi: 10.1038/sj.embor.7400916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Wang KW, Ilan N, Pausch MH. Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. J Mol Med. 1998;76:13–20. doi: 10.1007/s001090050186. [DOI] [PubMed] [Google Scholar]
- Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, Meadows HJ. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Honoré E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypolite JA, Lei Q, Chang S, Zderic SA, Butler S, Wein AJ, Malykhina AP, Chacko S. Spontaneous and evoked contractions are regulated by PKC-mediated signaling in detrusor smooth muscle: involvement of BK channels. Am J Physiol Renal Physiol. 2013;304:F451–F462. doi: 10.1152/ajprenal.00639.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Malykhina AP, Lupu F, Akbarali HI. Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G274–G285. doi: 10.1152/ajpgi.00472.2003. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, Horowitz B. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honoré E, Jodar M, Guy N, Lazdunski M, Patel AJ. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Pan XQ, Villamor AN, Asfaw TS, Chang S, Zderic SA, Malykhina AP. Lack of transient receptor potential vanilloid 1 channel modulates the development of neurogenic bladder dysfunction induced by cross-sensitization in afferent pathways. J Neuroinflammation. 2013;10:3. doi: 10.1186/1742-2094-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999a;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000a;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999b;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000b;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Lei Q, Chang S, Pan XQ, Villamor AN, Smith AL, Seftel AD. Bladder outlet obstruction triggers neural plasticity in sensory pathways and contributes to impaired sensitivity in erectile dysfunction. Am J Physiol Regul Integr Comp Physiol. 2013a;304:R837–R845. doi: 10.1152/ajpregu.00558.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Lei Q, Pan XQ, Greenwood-Van Meerveld B, Foreman RD. Differential effects of intravesical resiniferatoxin on excitability of bladder spinal neurons upon colon–bladder cross-sensitization. Brain Res. 2013b;1491:213–224. doi: 10.1016/j.brainres.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Monaghan K, Baker SA, Dwyer L, Hatton WC, Sik Park K, Sanders KM, Koh SD. The stretch-dependent potassium channel TREK-1 and its function in murine myometrium. J Physiol. 2011;589:1221–1233. doi: 10.1113/jphysiol.2010.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway RW, Petrou S, Kirber MT, Walsh JV, Jr, Singer JJ. Stretch activation of a toad smooth muscle K+ channel may be mediated by fatty acids. J Physiol. 1995;484:331–337. doi: 10.1113/jphysiol.1995.sp020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XQ, Gonzalez JA, Chang S, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol. 2010;225:262–273. doi: 10.1016/j.expneurol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honoré E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol. 2006;570:37–43. doi: 10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Anishkin A. Mechanosensitive channels: what can we learn from ‘simple’ model systems? Trends Neurosci. 2004;27:345–351. doi: 10.1016/j.tins.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Science's STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertyshnikova S, Knox RJ, Plym MJ, Thalody G, Griffin C, Neelands T, Harden DG, Signor L, Weaver D, Myers RA, Lodge NJ. BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: a putative potassium channel opener with bladder-relaxant properties. J Pharmacol Exp Ther. 2005;313:250–259. doi: 10.1124/jpet.104.078592. [DOI] [PubMed] [Google Scholar]
- Tichenor JN, Hansen ET, Buxton IL. Expression of stretch-activated potassium channels in human myometrium. Proc West Pharmacol Soc. 2005;48:44–48. [PubMed] [Google Scholar]
- Turner WH, Brading AF. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther. 1997;75:77–110. doi: 10.1016/s0163-7258(97)00038-7. [DOI] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Stretch-activated nonselective cation channels in urinary bladder myocytes: importance for pacemaker potentials and myogenic response. EXS. 1993;66:93–99. doi: 10.1007/978-3-0348-7327-7_6. [DOI] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994;480:439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Singer CA, Buxton IL. Variants of stretch-activated two-pore potassium channel TREK-1 associated with preterm labor in humans. Biol Reprod. 2012;87:96–99. doi: 10.1095/biolreprod.112.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]


