Abstract
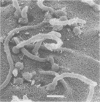
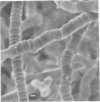
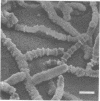
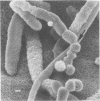
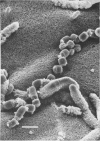
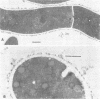
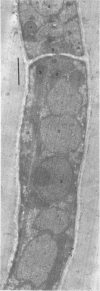
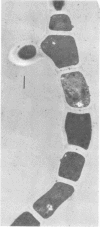
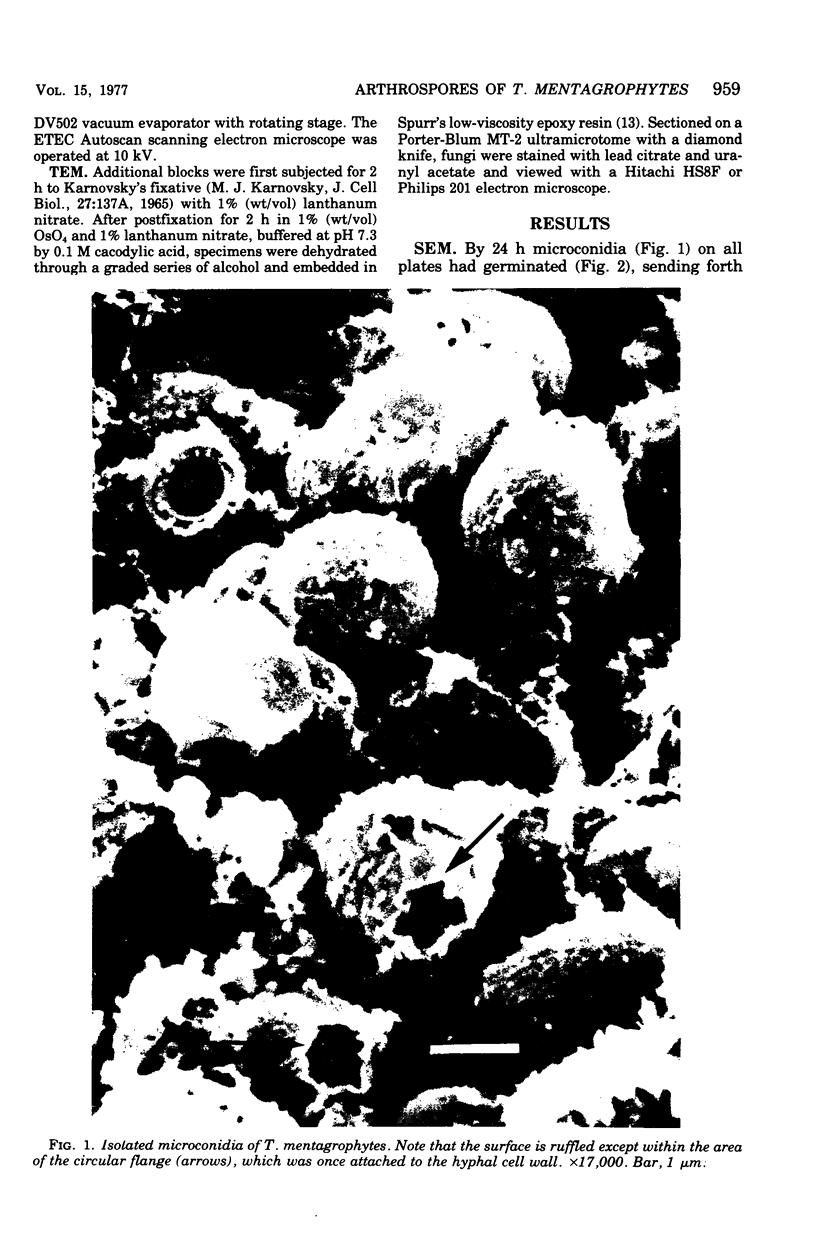
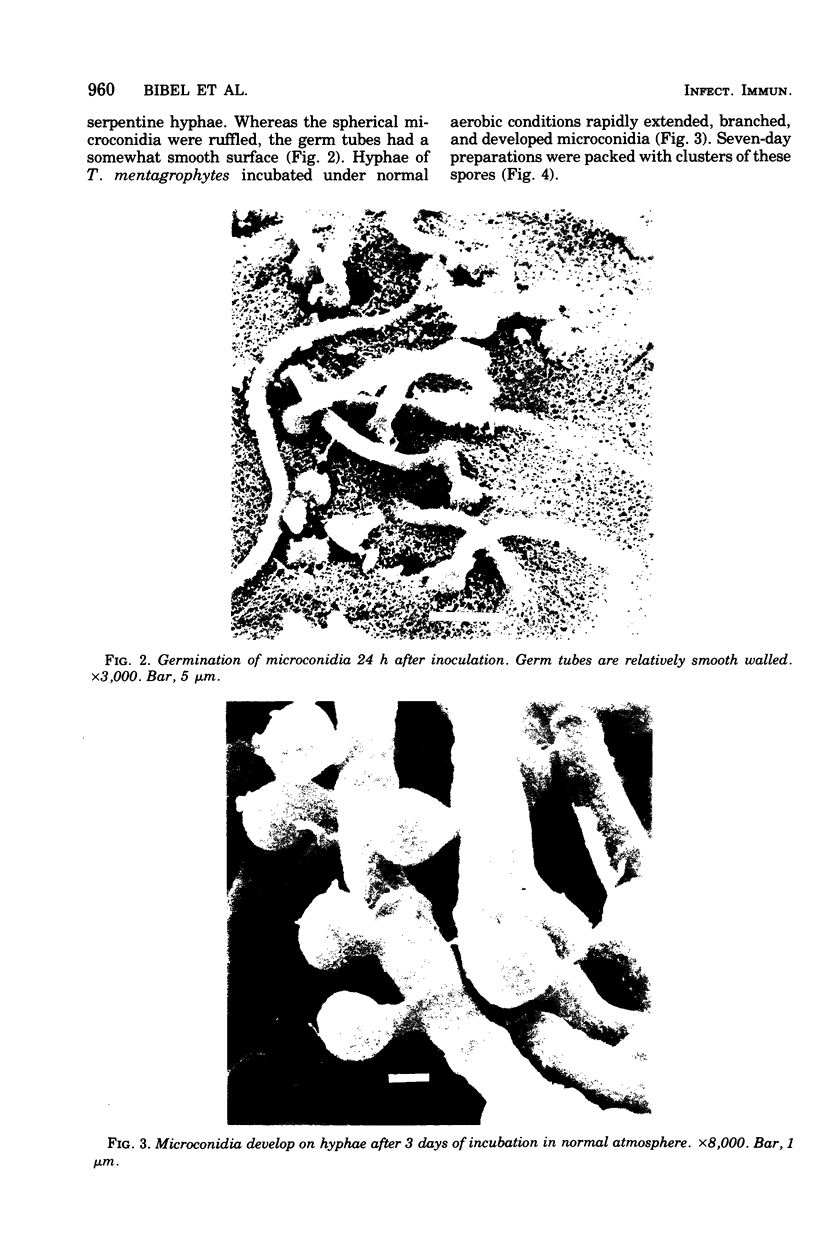
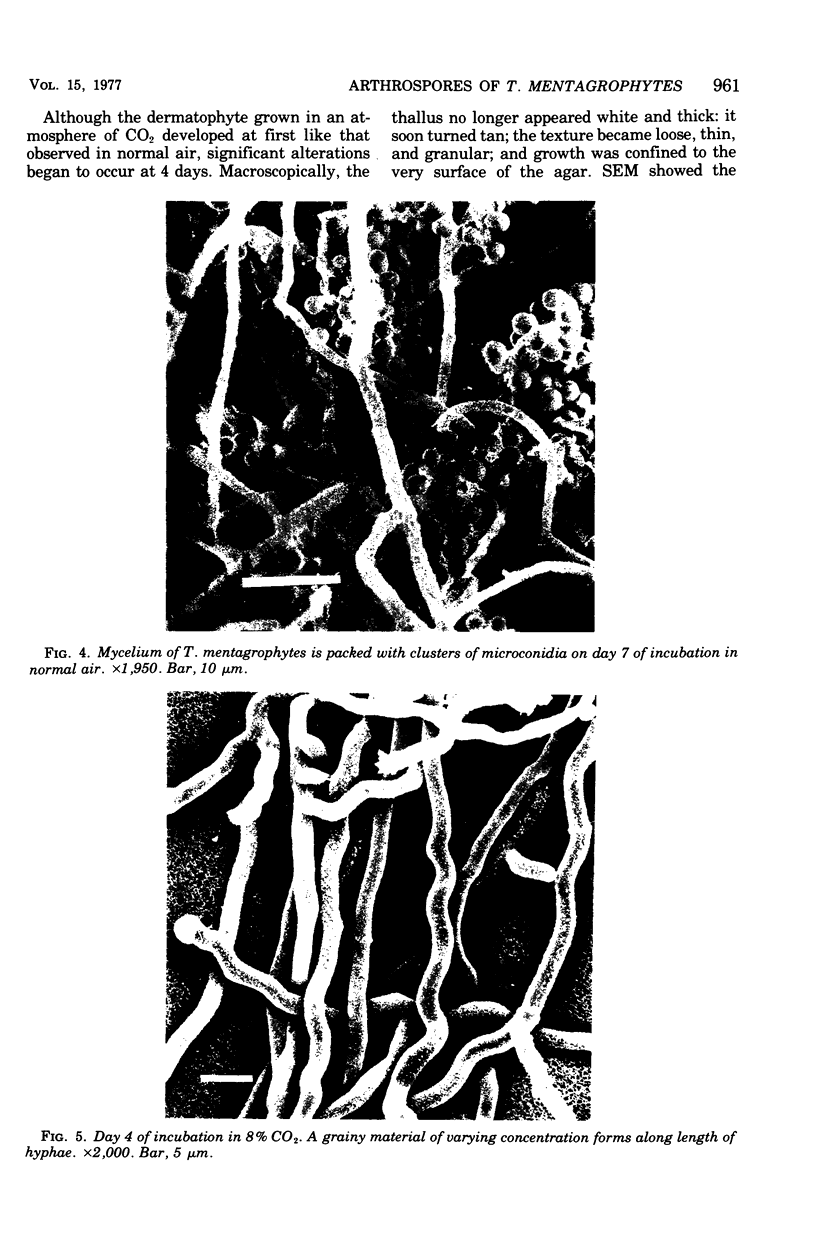
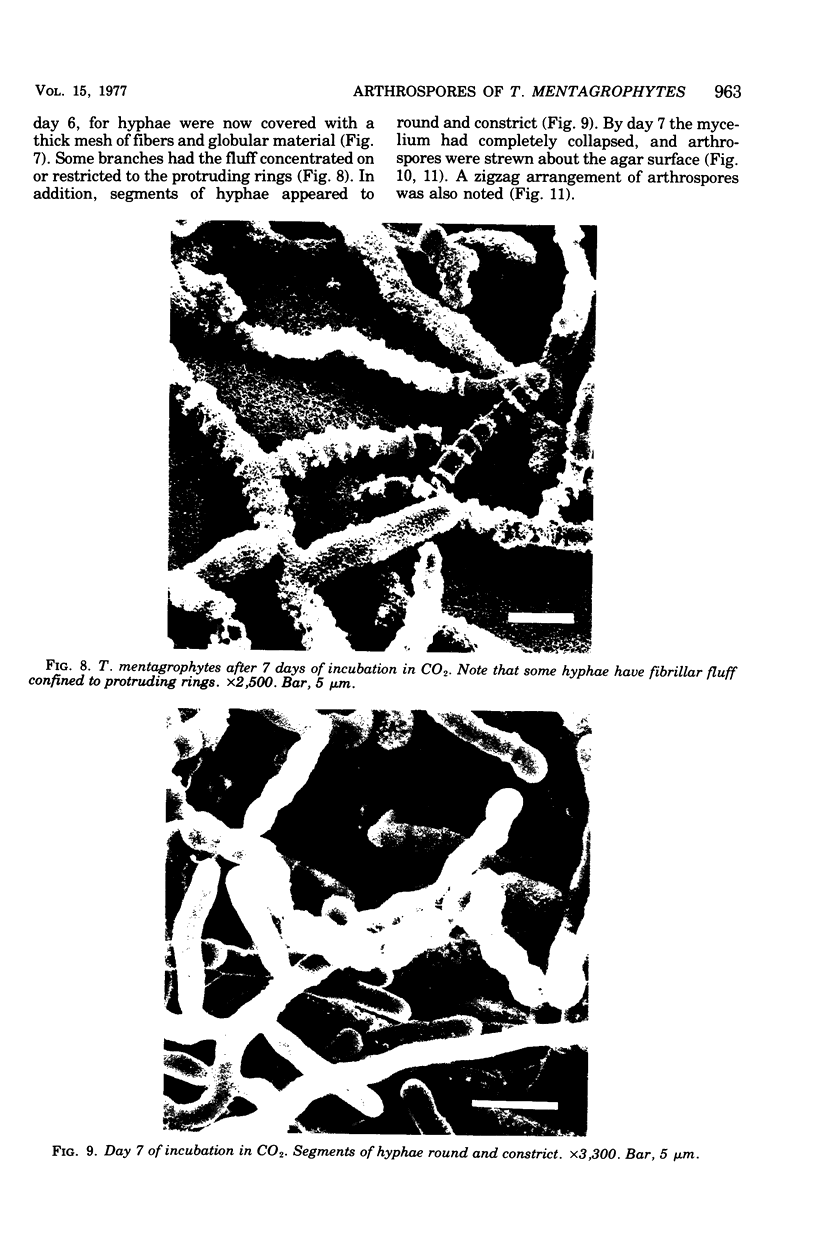
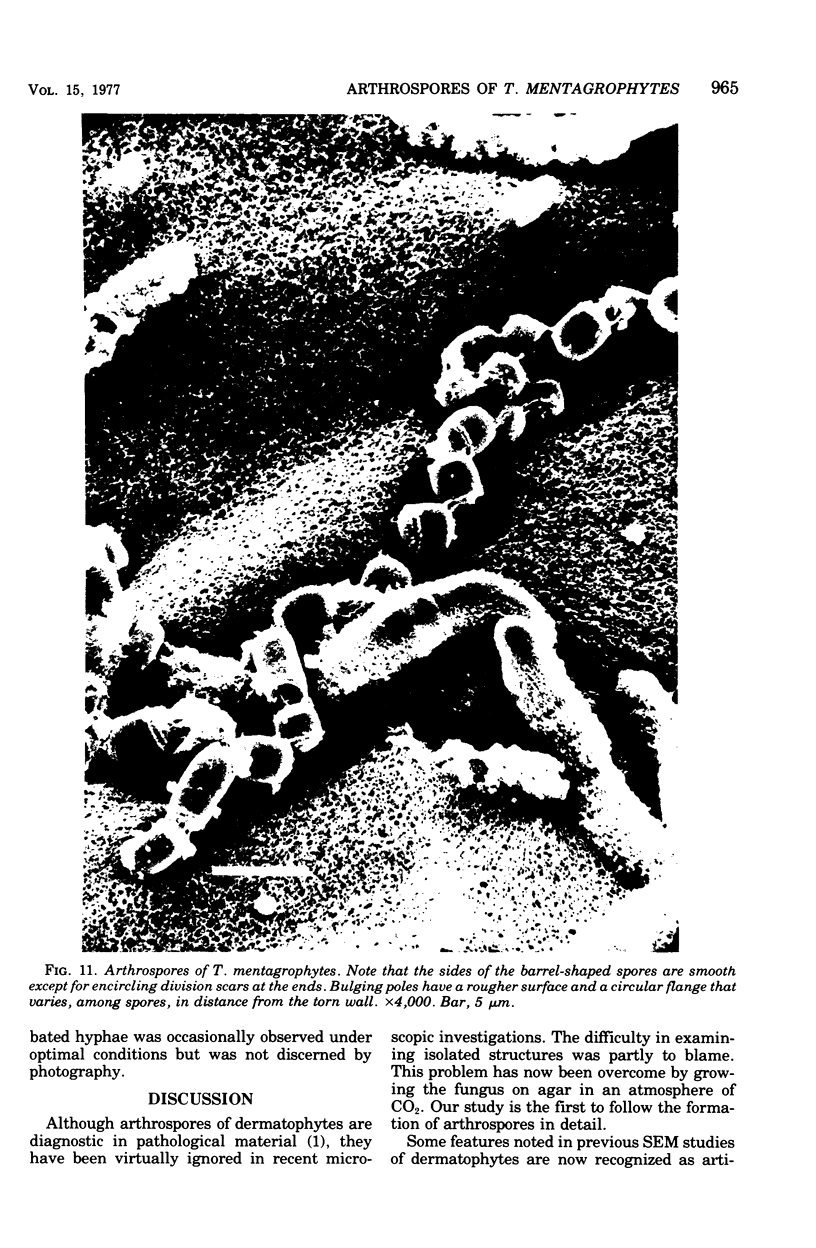
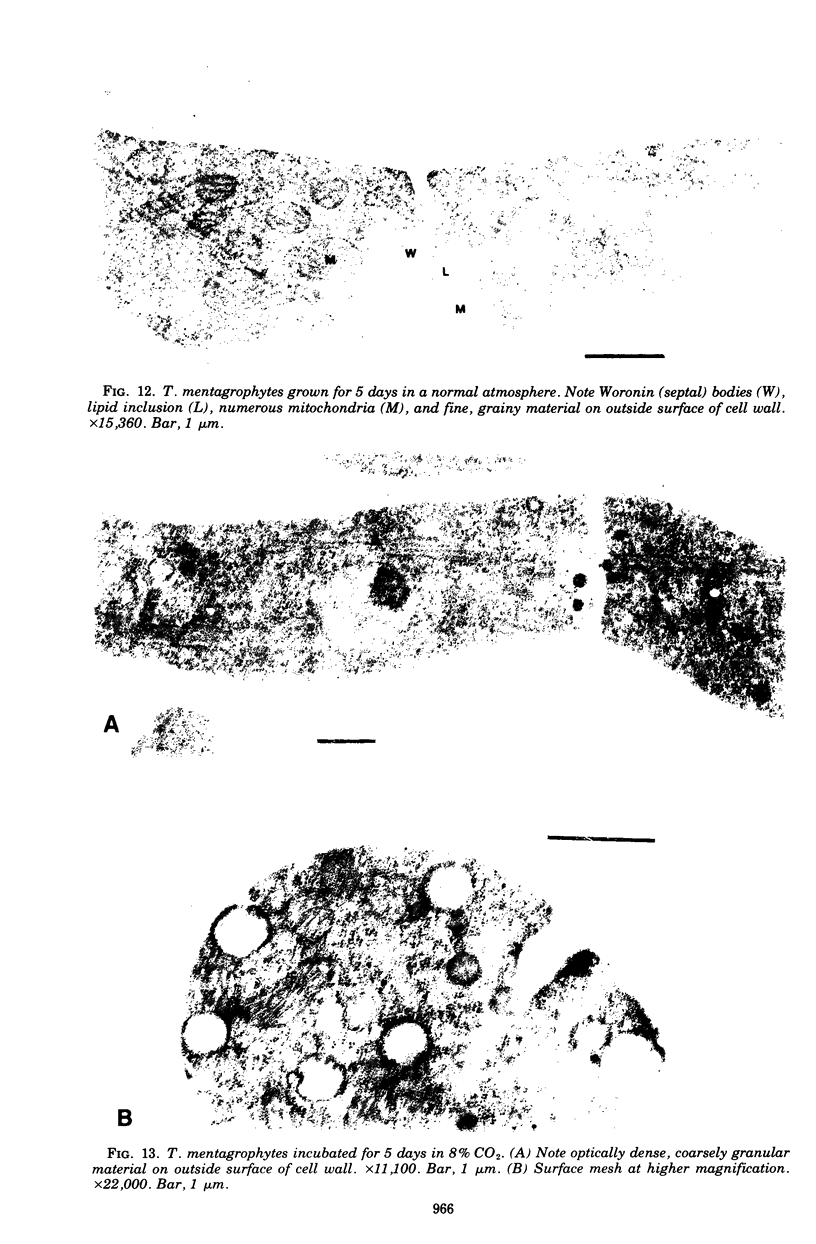
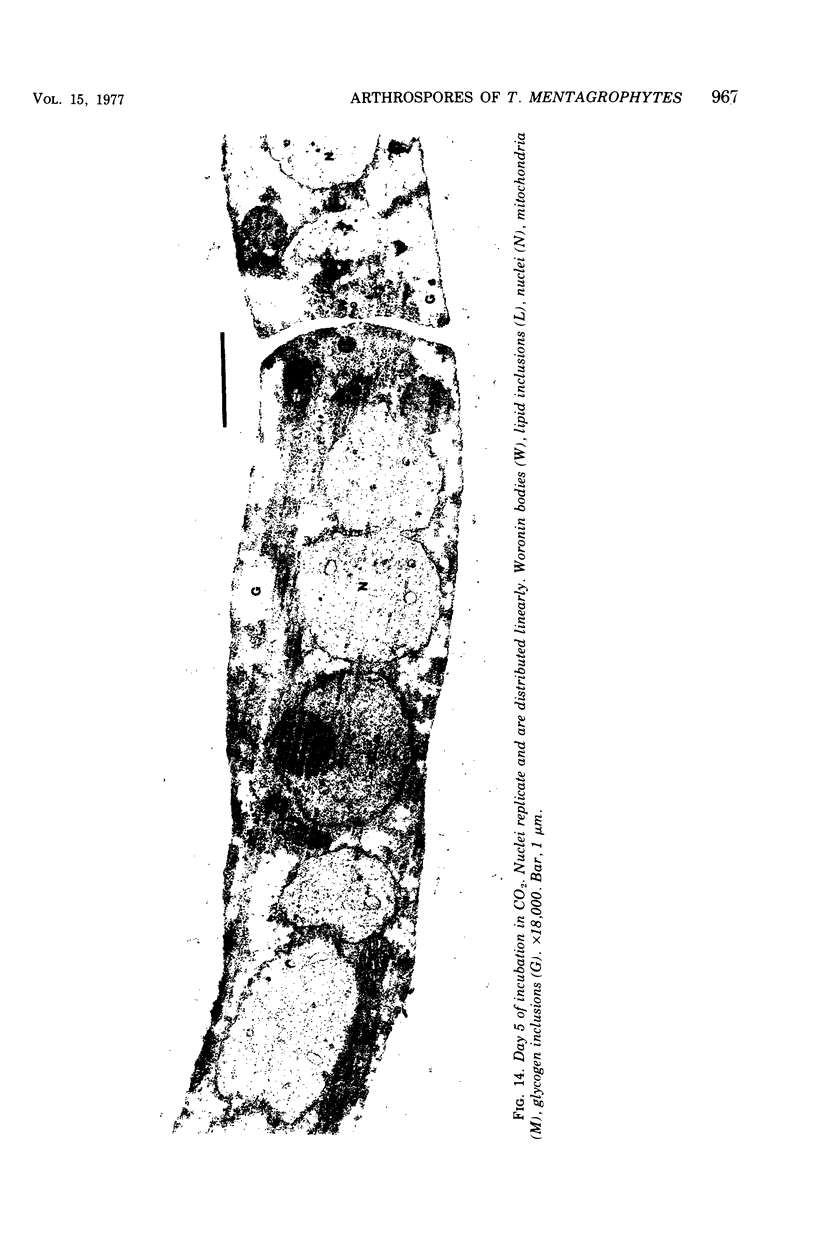
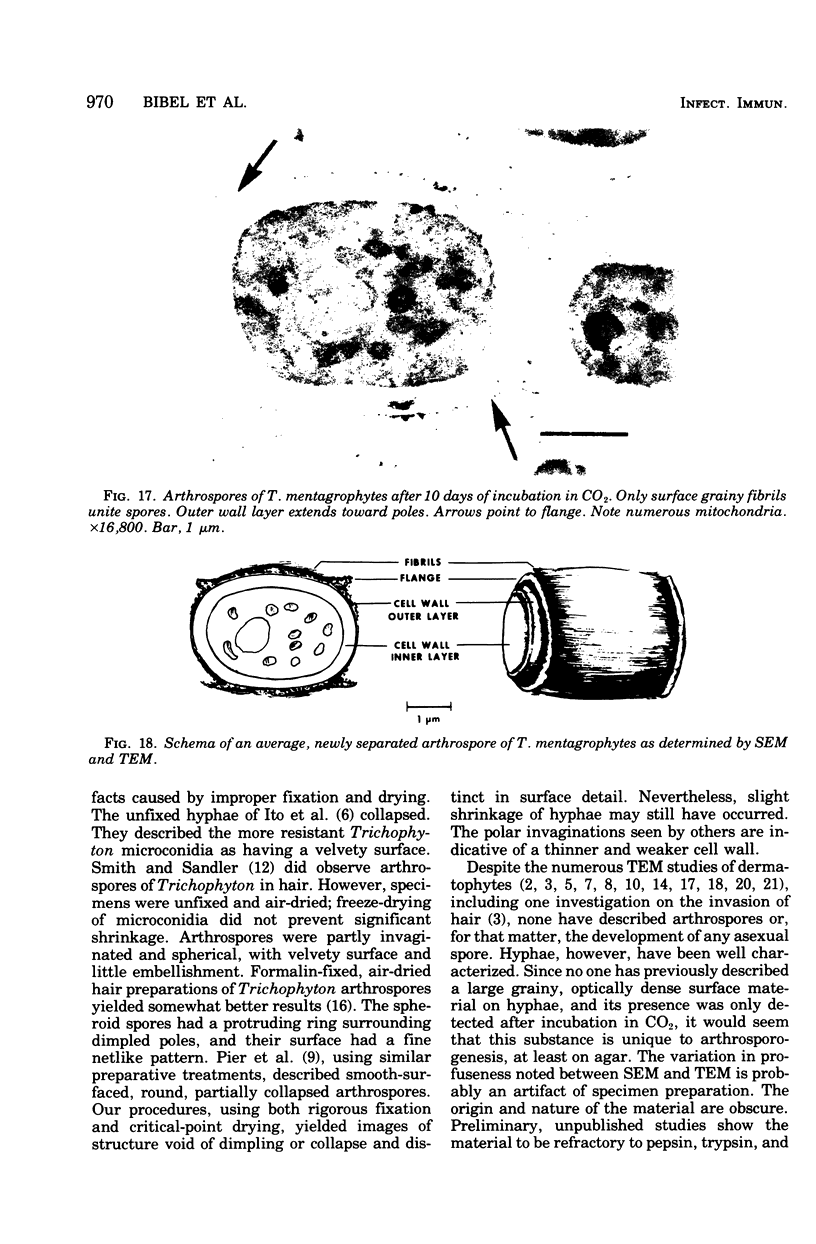
Arthrosporogenesis of the dermatophyte Trichophyton mentagrophytes was examined by light and by scanning and transmission electron microscopy. Sabouraud dextrose agar plates were inoculated with microconidia and incubated in an atmosphere of 8% CO2. Typical germination and hyphal branching continued to day 4, when hyphae began to be increasingly coated with granular-fibrillar material. Multiple replication of nuclei and formation of segregating septa followed. By day 6 the thick surface mesh sometimes was restricted to protruding rings, probably over septa. Between days 6 and 7, after thickening of outer and septal walls, units began to round and separate. Triangular gaps, which developed at the junction of septa and outer wall layers, enlarged so that spores were held together at their poles and along a tangential ring. With elongation of the spore to its barrel shape, the halves of the septum separated and the ring pulled apart, leaving a jagged, circular flange originating from the outer layer of cell wall extended toward the poles, covering the apparently exposed inner wall layer. Newly formed arthrospores, which measured 2.0 to 3.3 by 2.9 to 3.8 micronm and possessed walls of about 0.33-micronm thickness, has smooth sides but somewhat rough poles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Michaels G. E. Microsporum gypseum macroconidial development revealed by transmission and scanning electron microscopy. Sabouraudia. 1972 Mar;10(1):52–55. doi: 10.1080/00362177285190111. [DOI] [PubMed] [Google Scholar]
- Baxter M., Mann P. R. Electron microscopic studies of the invasion of human hair in vitro by three keratinophilic fungi. Sabouraudia. 1969 Feb;7(1):33–37. [PubMed] [Google Scholar]
- CHIN B., KNIGHT S. G. Growth of Trichophyton mentagrophytes and Trichophyton rubrum in increased carbon dioxide tensions. J Gen Microbiol. 1957 Jun;16(3):642–646. doi: 10.1099/00221287-16-3-642. [DOI] [PubMed] [Google Scholar]
- Hill T. W. Ultrastructure of ascosporogenesis in Nannizzia gypsea. J Bacteriol. 1975 May;122(2):743–748. doi: 10.1128/jb.122.2.743-748.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Nozawa Y., Suzuki H., Setoguti T. Surface structure of dermatophytes as seen by the scanning electron microscope. Sabouraudia. 1970 Feb;7(4):270–272. doi: 10.1080/00362177085190501. [DOI] [PubMed] [Google Scholar]
- Ito Y., Setoguti T., Nozawa Y., Sakurai S. An electron microscopic observation of Trichophyton violaceum. J Invest Dermatol. 1967 Feb;48(2):124–127. [PubMed] [Google Scholar]
- LADEN E. L., ERICKSON J. O. Electron microscope study of Epidermophyton floccosum. J Invest Dermatol. 1958 Jul;31(1):55–58. [PubMed] [Google Scholar]
- Pier A. C., Rhoades K. R., Hayes T. L., Gallagher J. Scanning electron microscopy of selected dermatophytes of veterinary importance. Am J Vet Res. 1972 Mar;33(3):607–613. [PubMed] [Google Scholar]
- Pock-Steen B., Kobayasi T. Ultrastructure of the hyphal wall and septum of Trichophyton mentagrophytes. J Invest Dermatol. 1970 Dec;55(6):404–409. doi: 10.1111/1523-1747.ep12260512. [DOI] [PubMed] [Google Scholar]
- Reinhardt J. H., Allen A. M., Gunnison D., Akers W. A. Experimental human Trichophyton mentagrophytes infections. J Invest Dermatol. 1974 Nov;63(5):419–422. doi: 10.1111/1523-1747.ep12676579. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Sandler W. J. The surface structure of saprophytic and parasitic dermatophyte spores. Mycopathol Mycol Appl. 1971 Feb 19;43(2):153–159. doi: 10.1007/BF02051715. [DOI] [PubMed] [Google Scholar]
- TAPLIN D., BLANK H. Microscopic morphology of Trichophyton rubrum. J Invest Dermatol. 1961 Dec;37:523–528. [PubMed] [Google Scholar]
- TSUKAHARA T., SATO A., OKADA R. ELECTRON MICROSCOPIC STUDIES ON THE CYTOLOGICAL STRUCTURE OF TRICHOPHYTON MENTAGROPHYTES. Jpn J Microbiol. 1964 Sep;8:85–96. [PubMed] [Google Scholar]
- Taplin D., Zaias N., Rebell G., Blank H. Isolation and recognition of dermatophytes on a new medium (DTM). Arch Dermatol. 1969 Feb;99(2):203–209. [PubMed] [Google Scholar]
- Tosti A., Villardita S., Fazzini M. L., Scalici R. Contribution to the knowledge of dermatophytic invasion of hair. An investigation with the scanning electron microscope. J Invest Dermatol. 1970 Aug;55(2):123–134. doi: 10.1111/1523-1747.ep12291637. [DOI] [PubMed] [Google Scholar]
- Urabe H., Izu T. The ultrastructure of Trichophyton and a double cell wall in the hypha. J Invest Dermatol. 1969 Jun;52(6):508–513. doi: 10.1038/jid.1969.86. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Observations of Microsporium canis with cryoscanning and scanning electron microscopy. Mycopathologia. 1975 Dec 23;57(2):73–76. doi: 10.1007/BF01365706. [DOI] [PubMed] [Google Scholar]
- Werner H. J., Catsulis C., Jolly H. W., Jr, Carpenter C. L., Jr Electron microscope observations of the fine structure of Microsporum gypseum. J Invest Dermatol. 1967 May;48(5):481–484. doi: 10.1038/jid.1967.74. [DOI] [PubMed] [Google Scholar]
- Werner H. J., Jolly H. W., Jr, Spurlock B. O. Electron microscope observations on the fine structure of Microsporum canis. J Invest Dermatol. 1966 Jan;46(1):130–134. doi: 10.1038/jid.1966.20. [DOI] [PubMed] [Google Scholar]