Abstract
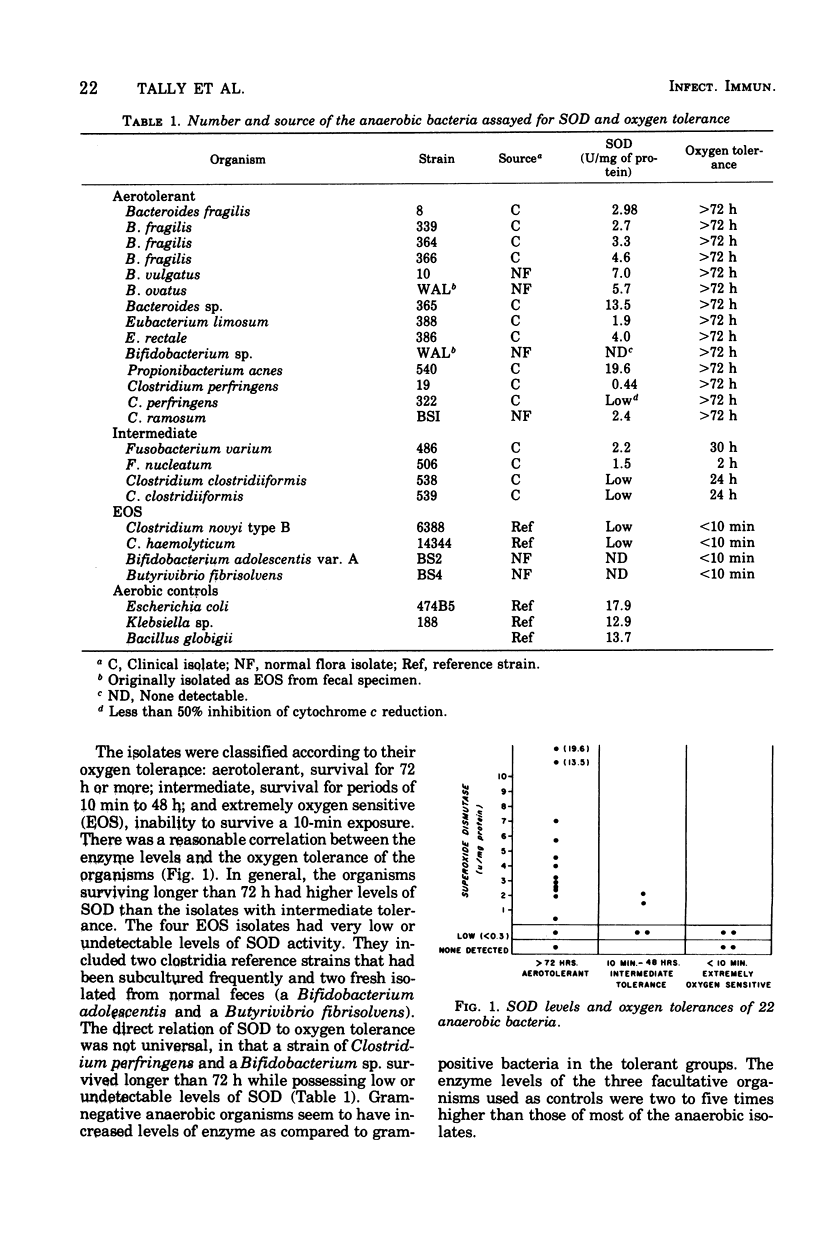
Twenty-two anaerobic bacteria isolated from infected sites and normal fecal flora were assayed for superoxide dismutase (SOD). The organisms were also classified according to their oxygen tolerance into aerotolerant, intermediate, and extremely oxygen-sensitive groups. There was a correlation between the enzyme level and the oxygen tolerance, in that the aerotolerant and intermediate organisms had SOD, whereas the extremely oxygen-sensitive isolates had low or undetectable enzyme. Among the oxygen-tolerant organisms, gram-negative bacteria had higher levels of SOD than gram-positive organisms. Oxygen was shown to induce SOD production in a strain of Bacteriodes fragilis grown in minimal medium under continuous-culture conditions. Enzyme levels in this isolate grown under static conditions were lower in minimal medium than in complex medium, indicating that other components in the complex medium were stimulating the production of SOD. Our data suggest that the variation in oxygen tolerance of anaerobes is usually related to their level of SOD. It is postulated that SOD may be a virulence factor that allows pathogenic anaerobes to survive in oxygenated tissues until the proper reduced conditions are established for their growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attebery H. R., Finegold S. M. Combined screw-cap and rubber-stopper closure for Hungate tubes (pre-reduced anaerobically sterilized roll tubes and liquid media). Appl Microbiol. 1969 Oct;18(4):558–561. doi: 10.1128/am.18.4.558-561.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attebery H. R., Sutter V. L., Finegold S. M. Effect of a partially chemically defined diet on normal human fecal flora. Am J Clin Nutr. 1972 Dec;25(12):1391–1398. doi: 10.1093/ajcn/25.12.1391. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Sullivan-Sigler N., Louie T. J., Gorbach S. L. Anaerobes survive in clinical specimens despite delayed processing. J Clin Microbiol. 1976 Feb;3(2):133–136. doi: 10.1128/jcm.3.2.133-136.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Morris J. G. Superoxide dismutase in some obligately anaerobic bacteria. FEBS Lett. 1975 Feb 15;50(3):315–318. doi: 10.1016/0014-5793(75)80518-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Superoxide dismutase in the anaerobic flagellates, Tritrichomonas foetus and Monocercomonas sp. J Biol Chem. 1974 Jul 25;249(14):4634–4637. [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Johnston J., Mayhew J. W., Gorbach S. L. Effect of dissolved oxygen and Eh and Bacteroides fragilis during continuous culture. Appl Environ Microbiol. 1976 Feb;31(2):168–172. doi: 10.1128/aem.31.2.168-172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Weinstein W. M., Sullivan N. M., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: quantitative bacteriology of infected animals. Infect Immun. 1974 Dec;10(6):1256–1259. doi: 10.1128/iai.10.6.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Fallon A., Finegold S. M. Comparison of methods for isolation of anaerobic bacteria from clinical specimens. Appl Microbiol. 1973 Jan;25(1):77–85. doi: 10.1128/am.25.1.77-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Stewart P. R., Sutter V. L., Rosenblatt J. E. Oxygen tolerance of fresh clinical anaerobic bacteria. J Clin Microbiol. 1975 Feb;1(2):161–164. doi: 10.1128/jcm.1.2.161-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


