Abstract
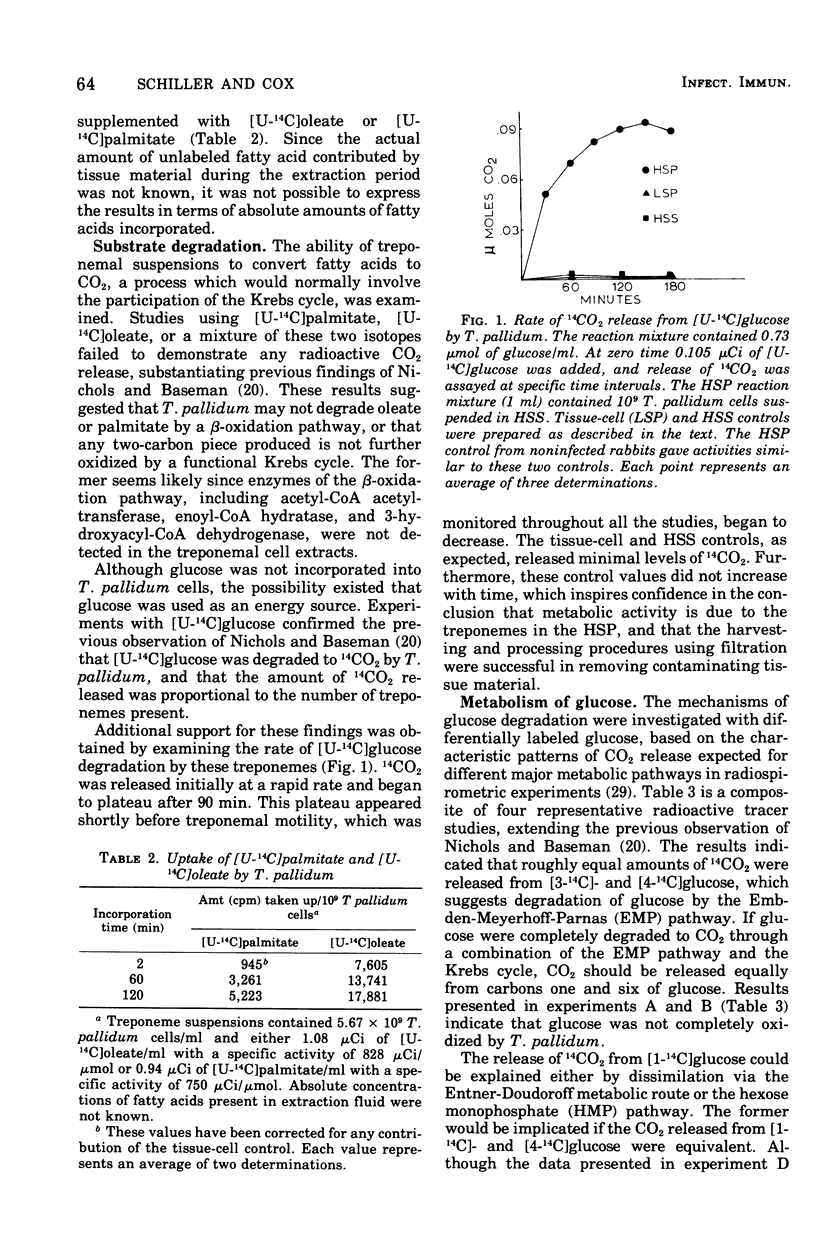
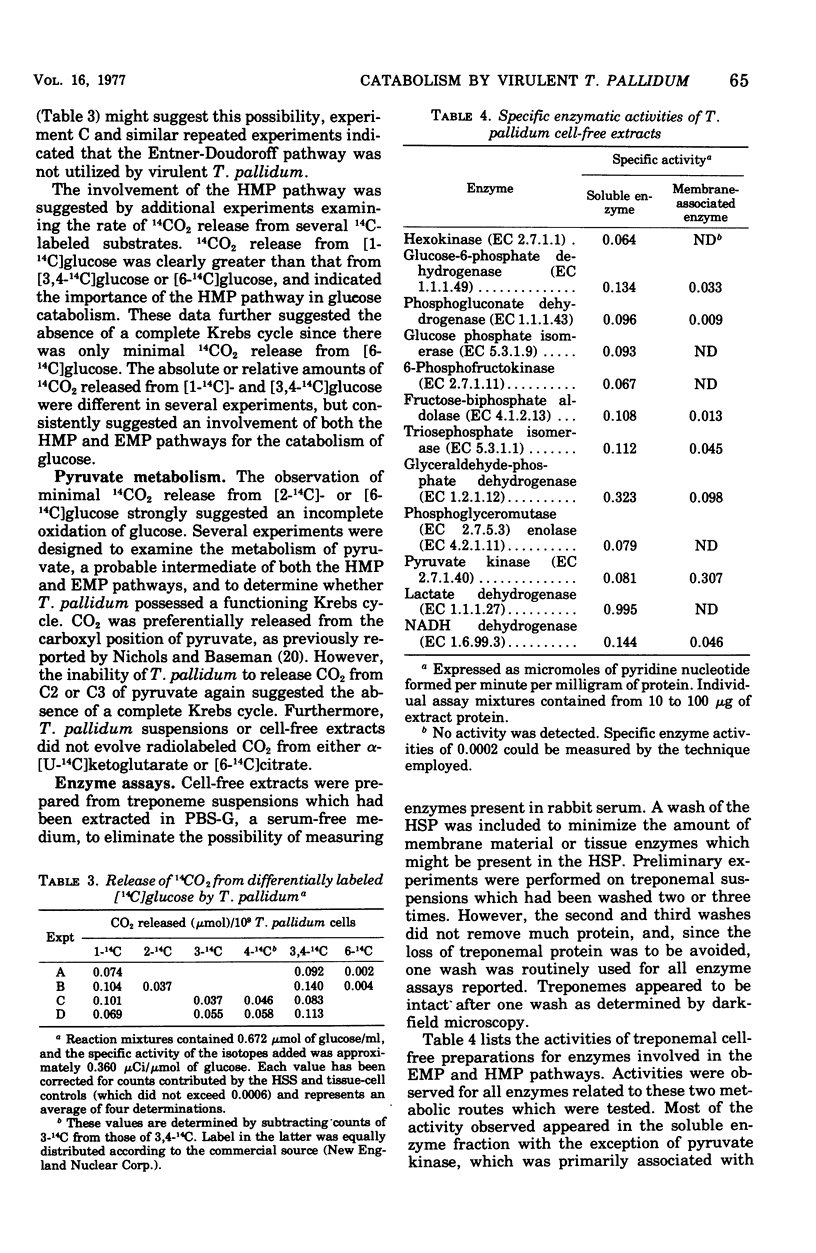
We describe a procedure which permits essentially full recovery of physiologically active Treponema pallidum from rabbit testicular extracts and greatly reduces contaminating tissue material. Such preparations were employed for investigations of the ability of T. pallidum to catabolize glucose and fatty acids. Radiorespirometric studies revealed that glucose and pyruvate, but not oleate or palmitate, could be degraded to CO2. The use of differentially labeled glucose, in conjunction with enzymatic analyses, indicated that glucose was catabolized by a combination of the Embden-Meyerhoff-Parnas and hexose monophosphate pathways. Pyruvate was degraded to CO2 from only the carboxyl position, suggesting the absence of a functioning Krebs cycle; this was substantiated by additional enzyme analyses and radiorespirometric experiments. Oleate and palmitate were incorporated but not catabolized by beta-oxidation. Glucose, although catabolized, was not incorporated. The potential significance of these findings is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Hayes N. C. Virulent Treponema pallidum: aerobe or anaerobe. Infect Immun. 1976 Mar;13(3):704–711. doi: 10.1128/iai.13.3.704-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. Purification of Treponema pallidum from Infected Rabbit Tissue: Resolution into Two Treponemal Populations. Infect Immun. 1974 Nov;10(5):1062–1067. doi: 10.1128/iai.10.5.1062-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler F. W., Jr, Clark J. W., Jr Passage of Treponema pallidium through membrane filters of various pore sizes. Appl Microbiol. 1970 Feb;19(2):326–328. doi: 10.1128/am.19.2.326-328.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Barber M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 1974 Jul;10(1):123–127. doi: 10.1128/iai.10.1.123-127.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Specific agglutination of Treponema pallidum by sera from rabbits and human beings with treponemal infections. J Exp Med. 1955 Apr 1;101(4):367–382. doi: 10.1084/jem.101.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. Beta-oxidation of fatty acids by Leptospira. Can J Microbiol. 1970 Jan;16(1):41–45. doi: 10.1139/m70-007. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATHLEV T., PFAU C. J. PURIFICATION OF THE PATHOGENIC TREPONEMA PALLIDUM BY DENSITY GRADIENT CENTRIFUGATION. Scand J Clin Lab Invest. 1965;17:130–134. doi: 10.1080/00365516509077298. [DOI] [PubMed] [Google Scholar]
- REEVES H. C., AJL S. Occurrence and function of isocitritase and malate synthetase in bacteria. J Bacteriol. 1960 Mar;79:341–345. doi: 10.1128/jb.79.3.341-345.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Clark J. W., Jr, Cline G. B., Anderson N. G., Russell H. Separation of Treponema pallidum from tissue substances by continuous-flow zonal centrifugation. Appl Microbiol. 1972 Apr;23(4):714–720. doi: 10.1128/am.23.4.714-720.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivett T. L., Meyer E. A. Citrate cycle and related metabolism of Listeria monocytogenes. J Bacteriol. 1971 Sep;107(3):770–779. doi: 10.1128/jb.107.3.770-779.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váczi L., Király K., Réthy A. Lipid composition of treponemal strains. Acta Microbiol Acad Sci Hung. 1966;13(1):79–84. [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER M. M. Factors influencing the in vitro survival of Treponema pallidum. Am J Hyg. 1960 May;71:401–417. doi: 10.1093/oxfordjournals.aje.a120123. [DOI] [PubMed] [Google Scholar]
- Williams F. R., Hager L. P. Crystalline flavin pyruvate oxidase from Escherichia coli. I. Isolation and properties of the flavoprotein. Arch Biochem Biophys. 1966 Sep 26;116(1):168–176. doi: 10.1016/0003-9861(66)90025-7. [DOI] [PubMed] [Google Scholar]


