Abstract
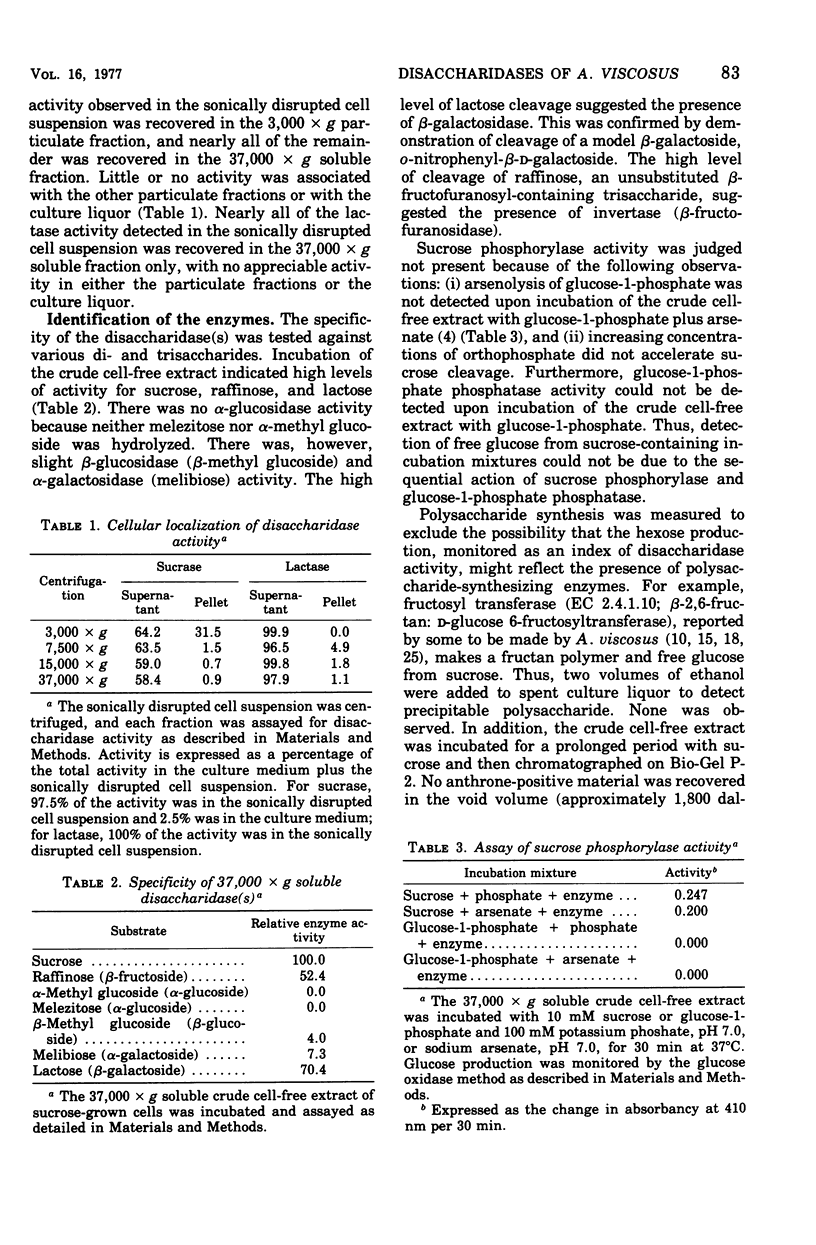
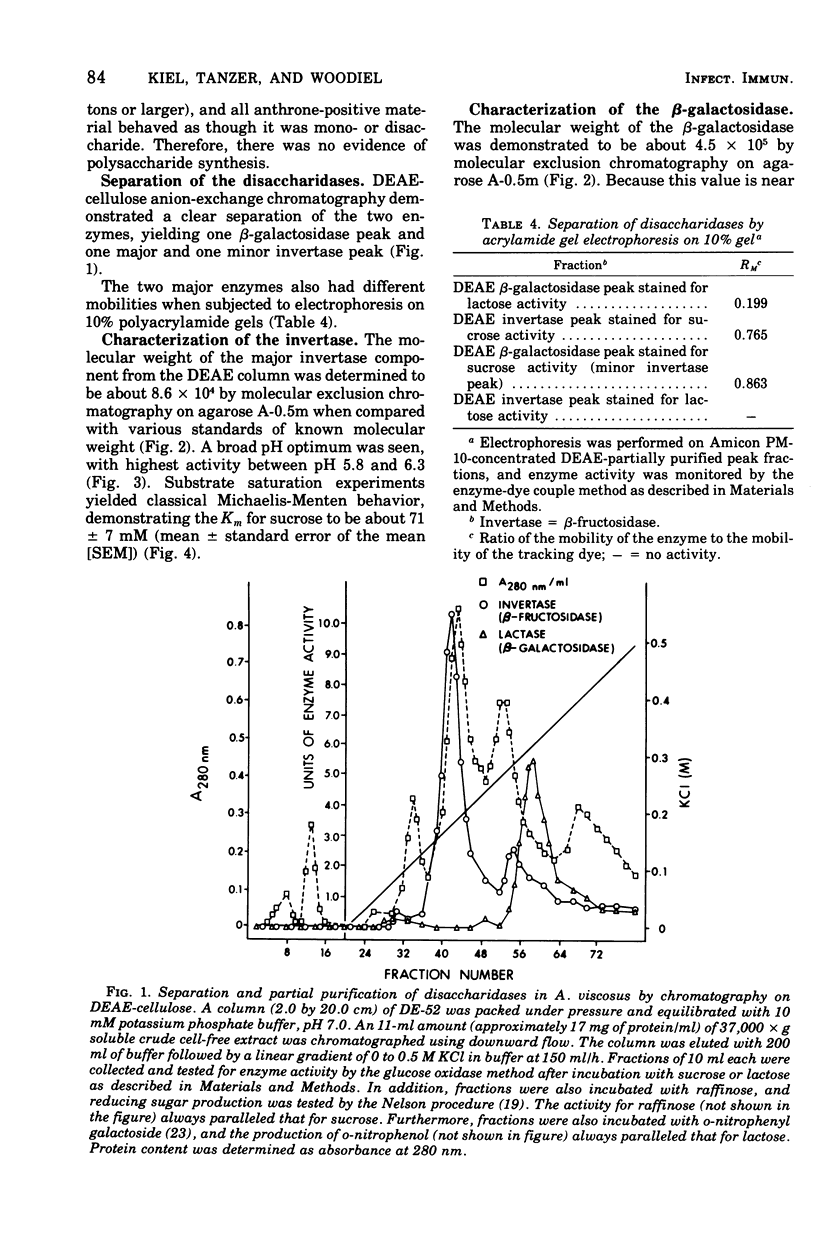
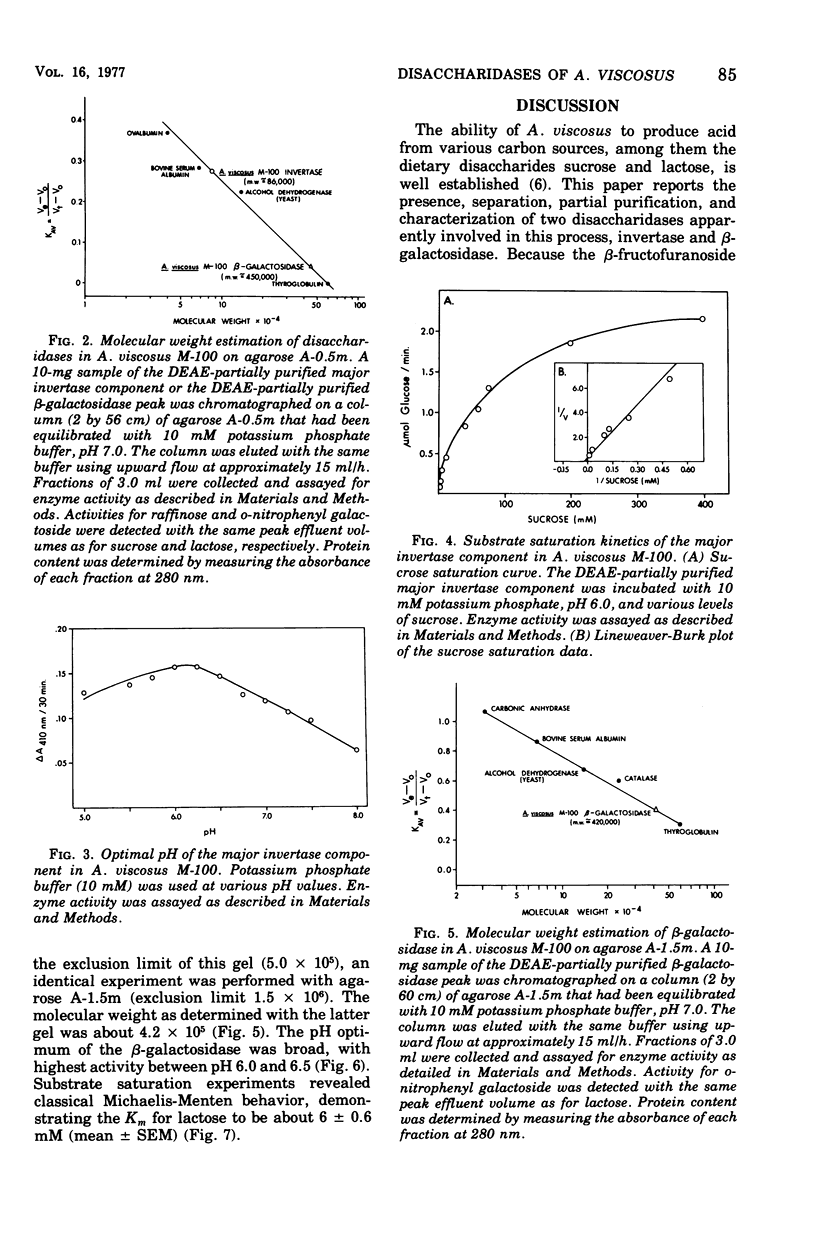
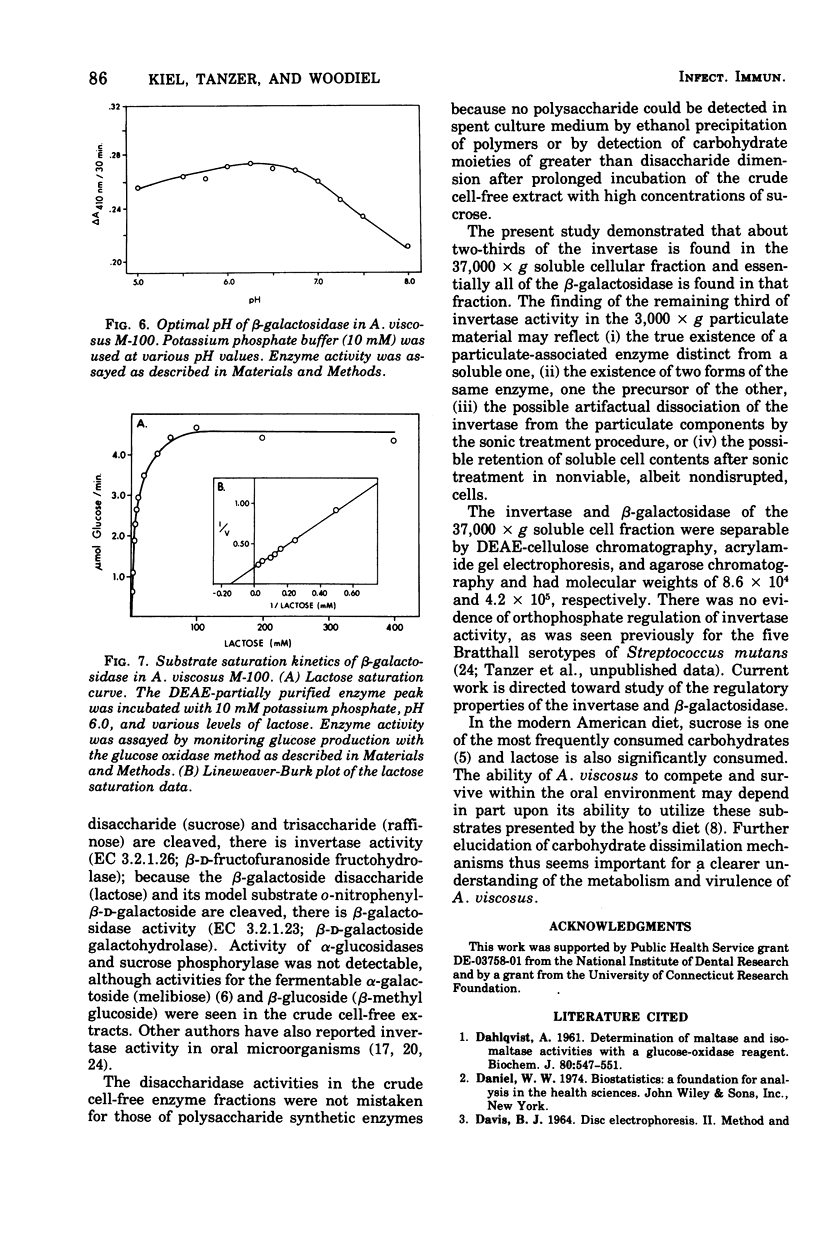
The initial step of disaccharide dissimilation by Actinomyces viscosus serotype 2 strain M-100 was studied. Sucrase activity was found in the 3,000 X g particulate fraction and the 37,000 X g soluble fraction of the cells, whereas lactase activity was found almost exclusively in the 37,000 X g soluble fraction. Neither sucrase nor lactase activity was appreciable in the culture liquor. Sucrose phosphorylase, alpha-glucosidase, and polysaccharide synthesis activities were not observed in the soluble cell fraction. The sucrase was identified as invertase (EC 3.2.1.26; beta-D-fructofuranoside fructohydrolase). The lactase was identified as beta-galactosidase (EC 3.2.1.23; beta-D-galactoside galactohydrolase). The enzymes in the 37,000 X g soluble fraction were separable by diethylamino-ethyl-cellulose chromatography, giving one beta-galactosidase peak and one major and one minor invertase peak. Acrylamide gel electrophoresis showed different electrophoretic mobilities of the enzymes. The molecular weight of the beta-galactosidase is about 4.2 X 10(5) and that of invertase is about 8.6 X 10(4). The beta-galactosidase has a Km for lactose of about 6 mM and a pH optimum between pH 6.0 and 6.5. The major invertase component has a Km for sucrose of about 71 mM and a pH optimum between pH 5.8 and 6.3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Identification of human strains of Actinomyces viscosus. Appl Microbiol. 1969 Jul;18(1):80–87. doi: 10.1128/am.18.1.80-87.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageage G. J., Johanssen I., Tanzer J. M. In vitro plaque formation by several oral diphtheroids implicated in periodontal disease. Infect Immun. 1970 Nov;2(5):683–685. doi: 10.1128/iai.2.5.683-685.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A., Jr A filamentous microorganism isolated from periodontal plaque in hamsters. 1. Isolation, morphology and general cultural characteristics. Sabouraudia. 1963 Oct;3(1):81–92. doi: 10.1080/00362176485190131. [DOI] [PubMed] [Google Scholar]
- Howell A., Jr, Jordan H. V. Production of an extracellular levan by Odontomyces viscosus. Arch Oral Biol. 1967 Apr;12(4):571–573. doi: 10.1016/0003-9969(67)90033-7. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Krichevsky M. I., Howell A., Jr, Lim S. Levan formation by Odontomyces viscosus. J Dent Res. 1969 Sep-Oct;48(5):938–942. doi: 10.1177/00220345690480055701. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C. H. Degradation of sucrose by whole cells and plaque of Actinomyces naeslundii. Infect Immun. 1974 Dec;10(6):1280–1291. doi: 10.1128/iai.10.6.1280-1291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Warner T. N., Palenik C. J., Somers P. J. Levan formation by whole cells of Actinomyces viscosus ATCC 15987. J Dent Res. 1975 Jul-Aug;54(4):906–906. doi: 10.1177/00220345750540043701. [DOI] [PubMed] [Google Scholar]
- Palenik C. J., Miller C. H. Extracellular invertase activity from Actinomyces viscosus. J Dent Res. 1975 Jan-Feb;54(1):186–186. doi: 10.1177/00220345750540011901. [DOI] [PubMed] [Google Scholar]
- Stephens R., DeBusk A. G. Beta-galactosidases from Neurospora crassa. Methods Enzymol. 1975;42:497–503. doi: 10.1016/0076-6879(75)42158-9. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven J. S., Vogels G. D., Bekkers M. F. A levansucrase from Actinomyces viscosus. Caries Res. 1976;10(1):33–48. doi: 10.1159/000260187. [DOI] [PubMed] [Google Scholar]


