Abstract
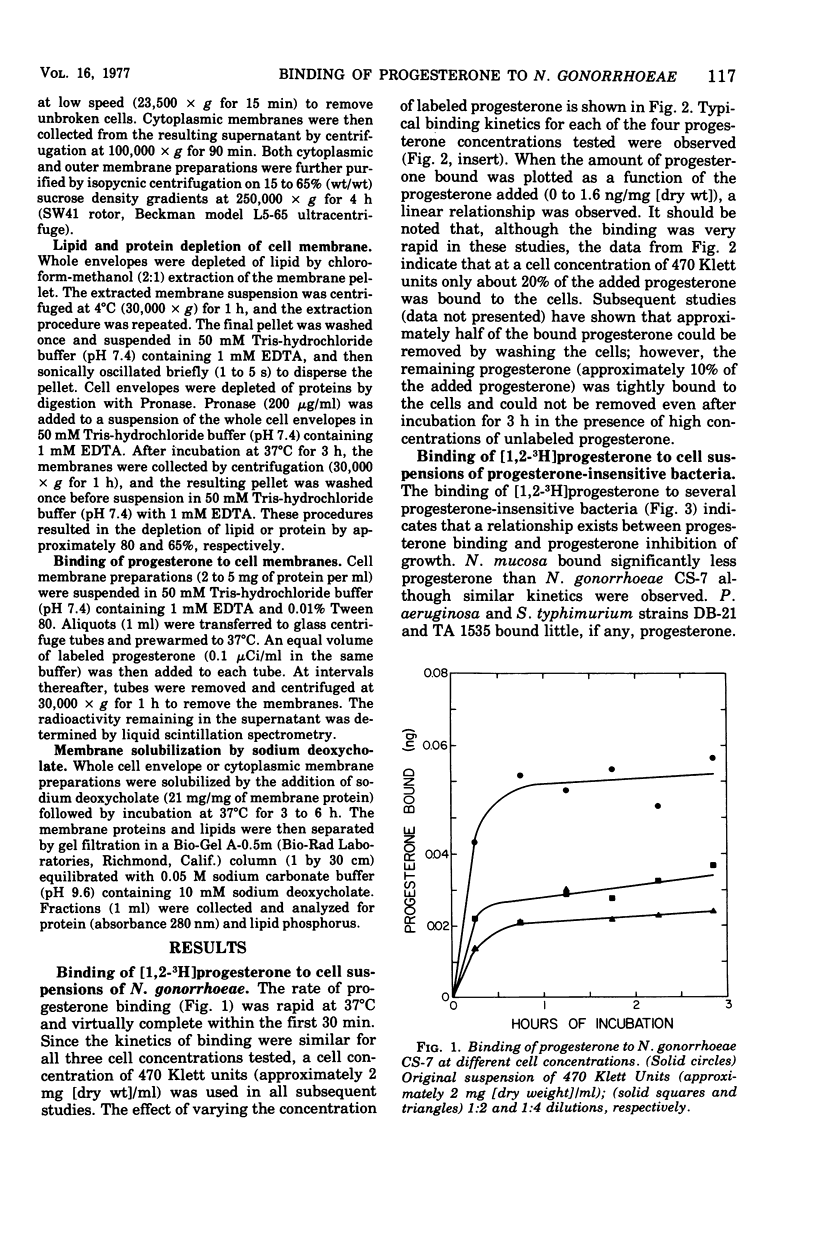
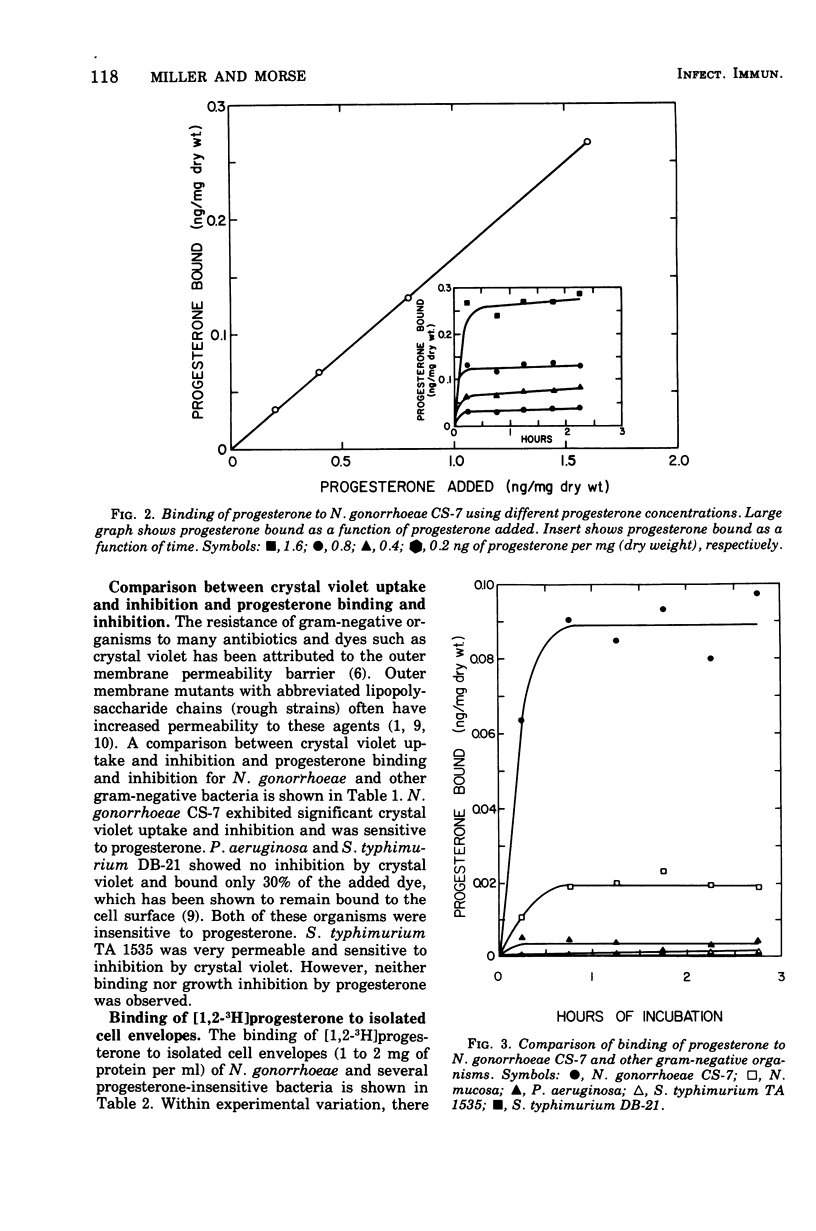
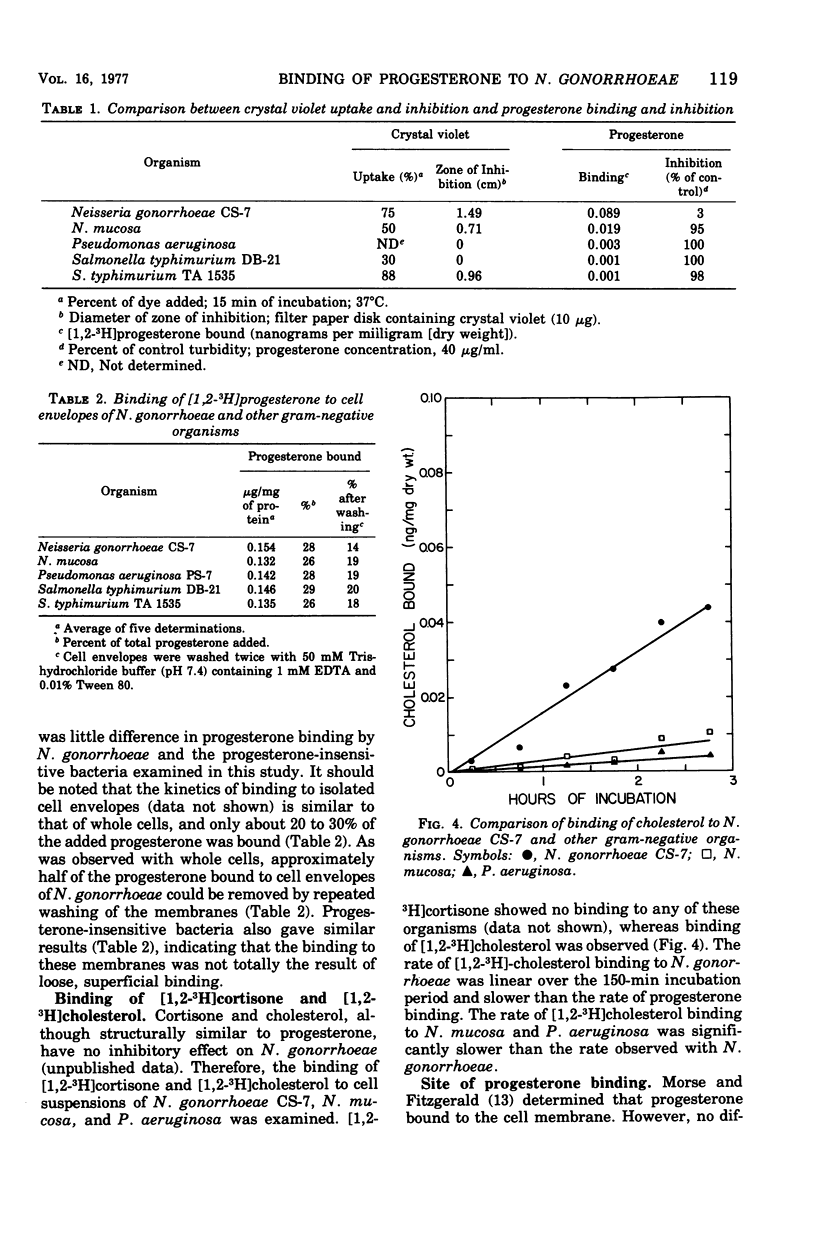
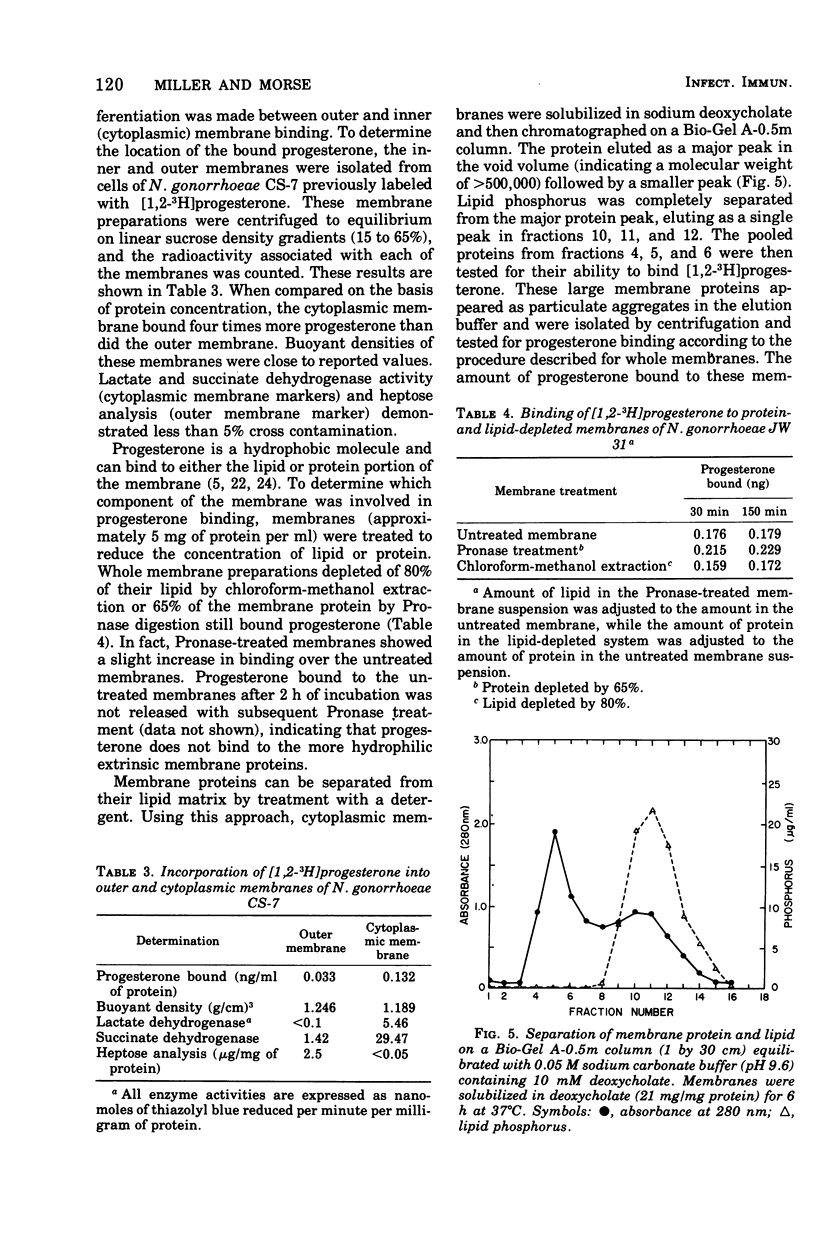
The binding of [1,2-3H]progesterone to progesterone-sensitive Neisseria gonorrhoeae CS-7 and the progesterone-insensitive Neisseria mucosa, Pseudomonas aeruginosa, and Salmonella typhimurium (rough and smooth strains) was investigated. The kinetics of binding to N. gonorrhoeae CS-7 demonstrated that the majority of the progesterone binding occurred and equilibrium was reached within the first 30 min. Despite the rapid binding of progesterone, only about 20% of the added steroid was bound at the cell concentration used throughout this study. Whole cells of progesterone-insensitive bacteria bound progesterone less efficiently than the progesterone-sensitive N. gonorrhoeae CS-7. N. mucosa bound low amounts of this steroid (20% of that bound by N. gonorrhoeae CS-7) whereas the other gram-negative bacteria exhibited little progesterone binding (<3% of that bound by N. gonorrhoeae CS-7). The outer membrane permeability of N. gonorrhoeae CS-7, as measured by crystal violet uptake and inhibition, was similar to the deep rough mutant of S. typhimurium TA 1535. The latter organism neither bound nor was inhibited by progesterone. However, isolated cell envelopes of N. gonorrhoeae and progesterone-insensitive bacteria all bound progesterone equally well. Cortisone and cholesterol, althouh structurally similar to progesterone, were not inhibitory to N. gonorrhoeae and did not bind to whole cells as well as progesterone. The major site of progesterone binding appeared to be the cytoplasmic membrane, which bound four times more progesterone than the outer membrane. In addition, isolated cytoplasmic membrane proteins bound more than three times more progesterone per milligram of protein than the intact membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Alexanian S., Schlecht S., Westphal O. Untersuchungen zur Wirkung von Kristallviolett auf Salmonella-R-Mutanten. 1. Bindungsversuche. Zentralbl Bakteriol Orig A. 1974 Feb;226(2):207–214. [PubMed] [Google Scholar]
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASAS-CAMPILLO C., BALANDRANO D., GALARZA A. Steroids. 159. Antimicrobial properties of 21,21-dimethoxy progesterone and other progesterone analogues. J Bacteriol. 1961 Mar;81:366–375. doi: 10.1128/jb.81.3.366-375.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavatti M., Michel G., Chataing B., Jouvenceaux A. Preservation of human erythrocyte membrane proteins during cold storage with addition of progesterone. Biochem Med. 1974 Mar;9(3):293–300. doi: 10.1016/0006-2944(74)90063-5. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Gershfeld N. L., Wormser M., Razin S. Cholesterol in mycoplasma membranes. I. Kinetics and equilibrium studies of cholesterol uptake by the cell membrane of Acholeplasma laidlawii. Biochim Biophys Acta. 1974 Jun 29;352(3):371–384. doi: 10.1016/0005-2736(74)90229-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Sparling P. F. Altered crystal violet permeability and lytic behavior in antibiotic-resistant and -sensitive mutants of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):757–763. doi: 10.1128/jb.124.2.757-763.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Cholesterol incorporation into bacterial membranes. J Bacteriol. 1975 Oct;124(1):570–572. doi: 10.1128/jb.124.1.570-572.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Wormser M., Gershfeld N. L. Cholesterol in mycoplasma membranes. II. Components of Acholeplasma laidlawii cell membranes responsible for cholesterol binding. Biochim Biophys Acta. 1974 Jun 29;352(3):385–396. doi: 10.1016/0005-2736(74)90230-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. The outer membrane of Proteus mirabilis. II. The extractable lipid fraction and electron-paramagnetic resonance analysis of the outer and cytoplasmic membranes. Biochim Biophys Acta. 1975 Feb 14;375(3):395–405. doi: 10.1016/0005-2736(75)90355-7. [DOI] [PubMed] [Google Scholar]
- SMITH R. F., SHAY D. E., DOORENBOS N. J. RELATIONSHIP OF SURFACTANT PROPERTIES OF SOME SYNTHETIC STEROIDS TO BACTERICIDAL ACTION. J Pharm Sci. 1964 Oct;53:1214–1216. doi: 10.1002/jps.2600531019. [DOI] [PubMed] [Google Scholar]
- Stoppani A. O., De Brignone C. M., Brignone J. A. Structural requirements for the action of steroids as inhibitors of electron transfer. Arch Biochem Biophys. 1968 Sep 20;127(1):463–475. doi: 10.1016/0003-9861(68)90251-8. [DOI] [PubMed] [Google Scholar]
- Varricchio F., Dorrenbos N. J., Stevens A. Effect of azasteroids on gram-positive bacteria. J Bacteriol. 1967 Feb;93(2):627–635. doi: 10.1128/jb.93.2.627-635.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F., Sanadi D. R. Inhibition of mitochondrial respiration by progesterone and an azasteroid. Arch Biochem Biophys. 1967 Jul;121(1):187–193. doi: 10.1016/0003-9861(67)90023-9. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Normark S., Bloom G. D. Rapid method for isolation of large quantities of outer membrane from Escherichia coli K-12 and its application to the study of envelope mutants. J Bacteriol. 1973 Sep;115(3):1191–1197. doi: 10.1128/jb.115.3.1191-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yielding K. L., Tomkins G. M. INHIBITION OF THE ENZYMIC OXIDATION OF DPNH BY STER HORMONES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1730–1739. doi: 10.1073/pnas.45.12.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotis W., Stanke R. Bacteriostatic action of progesterone on staphylococci and other microorganisms. J Bacteriol. 1966 Nov;92(5):1285–1289. doi: 10.1128/jb.92.5.1285-1289.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotis W., Waner J. Antimicrobial properties of testosterone and its intermediates. Antonie Van Leeuwenhoek. 1968;34(3):275–286. doi: 10.1007/BF02046449. [DOI] [PubMed] [Google Scholar]


