Abstract
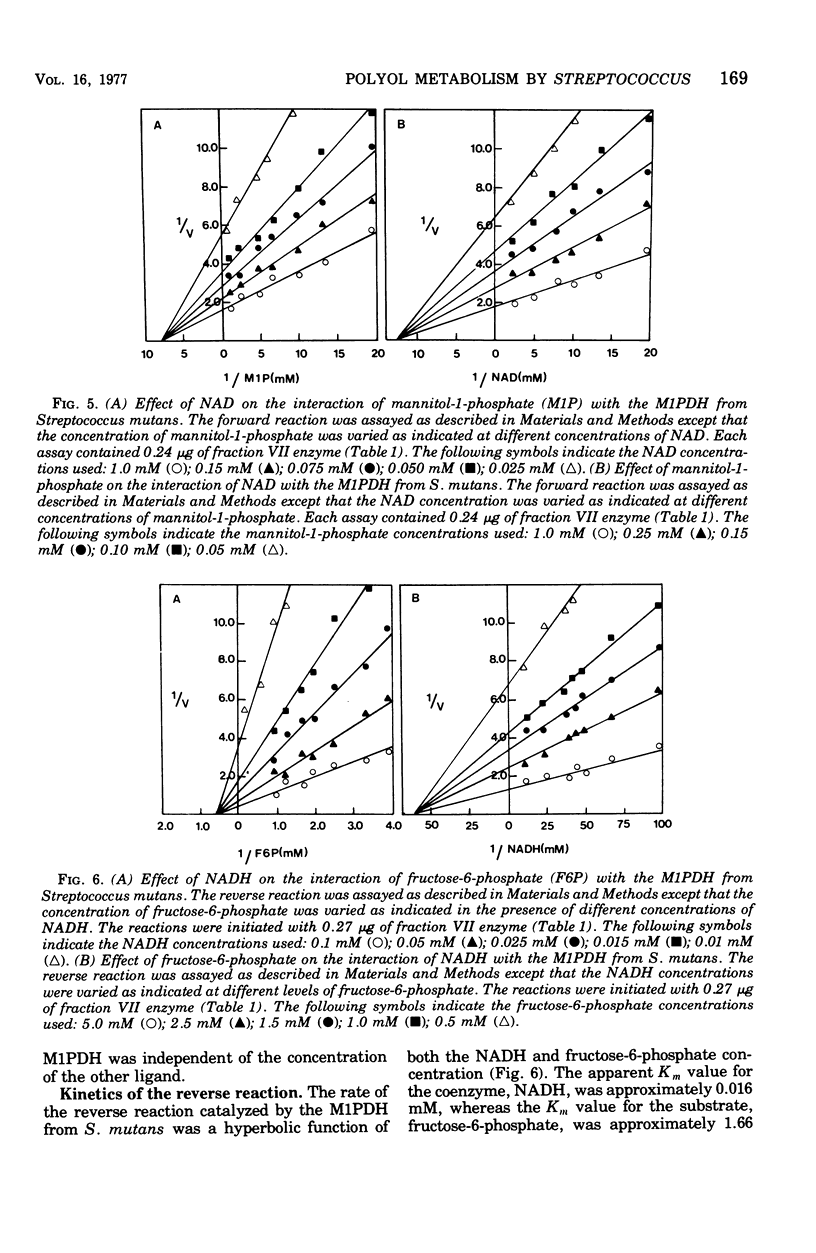
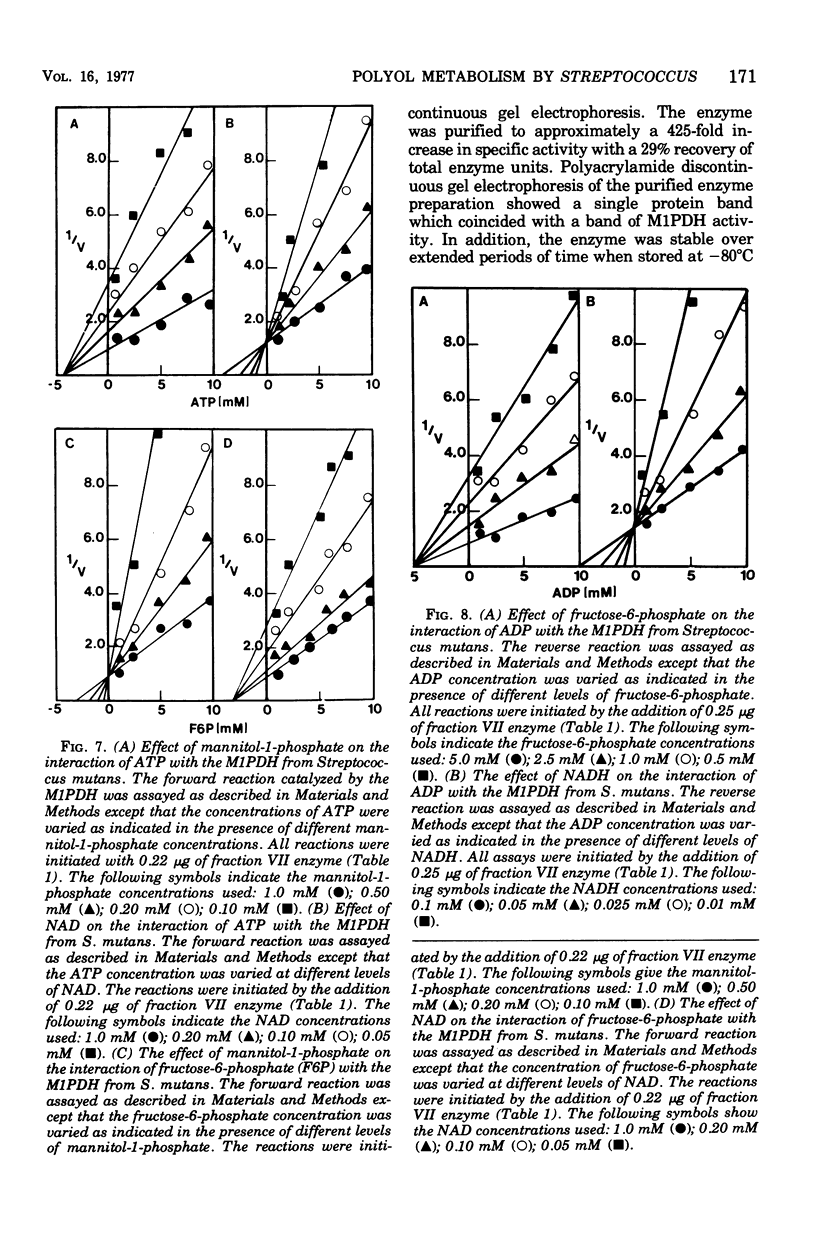
The mannitol-1-phosphate dehydrogenase (M1PDH) (EC 1.1.1.17) from Streptococcus mutans strain FA-1 was purified to approximately a 425-fold increase in specific activity with a 29% recovery of total enzyme units, using a combination of (i) streptomycin sulfate and ammonium sulfate precipitation and (ii) diethyl-aminoethyl-cellulose (DE-52), agarose A 0.5M, and agarose-nicotinamide adenine dinucleotide (NAD) affinity column chromatography. Polyacrylamide gel electrophoresis of the purified enzyme preparation showed a single protein component that coincided with a band of M1PDH activity. The enzyme had a molecular weight of approximately 45,000 and was stable for long periods of time when stored at −80°C in the presence of β-mercaptoethanol. Its activity was not affected by mono- or divalent cations, and high concentrations of ethylenedia-minetetraacetic acid were not inhibitory. The M1PDH catalyzed both the NAD-dependent oxidation of mannitol-1-phosphate and the reduced NAD (NADH)-dependent reduction of fructose-6-phosphate. The forward reaction was highly specific for mannitol-1-phosphate and NAD, whereas the reverse reaction was highly specific for NADH and fructose-6-phosphate. The Km values for mannitol-1-phosphate and NAD were 0.15 and 0.066 mM, respectively, and the Km values for fructose-6-phosphate and NADH were 1.66 and 0.016 mM, respectively. The forward and reverse reactions catalyzed by the M1PDH from S. mutans appeared to be under cellular control. Both adenosine 5′-triphosphate and fructose-6-phosphate were negative effectors of the forward reaction, whereas adenosine 5′-diphosphate served as a negative effector of the reverse reaction catalyzed by the enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Induction and regulation of a nicotinamide adenine dinucleotide-specific 6-phosphogluconate dehydrogenase in Streptococcus faecalis. J Bacteriol. 1972 Jan;109(1):106–115. doi: 10.1128/jb.109.1.106-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):117–126. doi: 10.1016/0003-9969(73)90026-5. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J Bacteriol. 1971 May;106(2):456–467. doi: 10.1128/jb.106.2.456-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. The caries-inducing property of variants of Streptococcus mutans. Odontol Revy. 1970;21(2):153–157. [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- MIZUSHIMA S., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. II. INTRACELLULAR CONCENTRATIONS OF GLYCOLYTIC INTERMEDIATES IN GLUCOSE-METABOLIZING WASHED CELLS. J Bacteriol. 1964 Jun;87:1429–1435. doi: 10.1128/jb.87.6.1429-1435.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]