Abstract
Inflammation in early human immunodeficiency virus type 1 (HIV-1) disease progression is not well characterized. Ninety patients with untreated primary HIV-1 infection were studied to determine associations of inflammatory proteins with early disease progression. High plasma tumor necrosis factor α (TNF-α) levels (≥8.5 pg/mL) were significantly associated with an increased viral load set point and shorter times to reaching a CD4+ T-cell count of <500 cells/mm3 and initiating antiretroviral therapy. The increased risk of reaching a CD4+ T-cell count of <500 cells/mm3 in the group with high TNF-α levels was driven by viral load but was independent of concurrent CD4+ T-cell count. Thus, TNF-α appears to be an important mediator of inflammation in patients with poor viral control and early HIV-1 disease progression.
Keywords: TNF-α, HIV/AIDS, inflammation, disease progression, viral load set point
Inflammation is a hallmark of human immunodeficiency virus type 1 (HIV-1) disease [1, 2]. During the acute phase of HIV-1 infection, there is generalized immune activation and production of many inflammatory cytokines, described as a cytokine storm [3]. Viral load subsequently declines, but it is thought that the ongoing immune activation and viral replication are associated with subsequent HIV-1 disease pathology [4].
The role of inflammation in HIV-1 pathogenesis was broadly recognized in the Strategies for Management of Antiretroviral Therapy (SMART) trial, which showed that elevated interleukin 6 (IL-6) and D-dimer levels were associated with increased all-cause mortality [5]. Subsequent studies have confirmed this finding, and associations have been reported with levels of C-reactive protein (CRP), soluble CD14, and lipopolysaccharide, among others [2, 6, 7]. Currently, the role of inflammation during primary HIV-1 infection (ie, after the acute phase and during establishment of the viral load set point) and in early disease progression is poorly understood.
We hypothesized that inflammation during early HIV-1 infection may be associated with subsequent clinical measures of disease progression, such as CD4+ T-cell count decline and antiretroviral therapy (ART) initiation. Therefore, we analyzed a number of well-described inflammatory cytokines and activation proteins in a cohort of patients with untreated primary HIV-1 infection. We report that plasma tumor necrosis factor α (TNF-α) levels during primary HIV-1 infection were correlated with viral control and associated with time to clinical end points. Furthermore, high TNF-α levels were associated with an increased risk of reaching a CD4+ T-cell count of <500 cells/mm3, and this risk was dependent on viral load but not on concurrent CD4+ T-cell count at the time of inflammatory protein analysis, suggesting an important role for virus-induced inflammation in early HIV-1 disease progression.
METHODS
Study Subjects
A total of 317 subjects from the Boston Acute and Early HIV-1 Study at Massachusetts General Hospital were evaluated for this study. Detailed demographic and clinical description of this cohort has been reported elsewhere [8]. The following inclusion criteria were used: no ART in first 2 months after enrollment, >6 months of follow-up with CD4+ T-cell count and viral load testing, and sufficient HIV-1 load data to calculate viral load set point. Ninety of 317 subjects met these criteria and were included in subsequent testing. The study was approved by the institutional review board, and informed consent was obtained for all subjects. The study was conducted in accordance with human experimentation guidelines of Massachusetts General Hospital and the Department of Health and Human Services.
Study Design
Study data were captured using REDCap (Research Electronic Data Capture) [9]. Clinical data and laboratory results were recorded at each visit. A retrospective analysis was conducted to determine the primary end points of (1) viral load set point and (2) days to reach a CD4+ T-cell count of <500 cells/mm3, as well as the secondary end points of (1) days to reach a CD4+ T-cell count of <350 cells/mm3 and (2) days to initiation of antiretroviral therapy (ART). Initiation of ART was clinically guided and decided by the patient's physician independently of the study. The time to each end point was calculated relative to the date of testing for inflammatory proteins.
Determination of Viral Load Set Point
Viral load set point was defined using the previously described algorithm by Fellay et al [10]. Briefly, viral load measurements obtained between 6 weeks and 1 year after enrollment were averaged (median, 3 measurements; range, 2–6 measurements). Each value was individually reviewed (by S. A. V.), and values were excluded if they were ±2 SDs from the mean, if the subject was receiving ART, or on the basis of clinical data, if the subject was determined to be in the acute phase of infection.
Cytokine and Plasma Protein Testing
TNF-α, interferon γ (IFN-γ), interleukin 1β (IL-1β), and interleukin 6 (IL-6) levels in frozen plasmas samples were measured using the Human ProInflammatory I 4-Plex Assay Ultra-Sensitive Kit from MSD (Gaithersburg, MD). Assays were performed according to the manufacturer's instructions. The CRP level was measured by a high-sensitivity standard immunochemiluminometric assay. The D-dimer level was measured by an automated quantitative enzyme-linked fluorescent assay. All laboratories performing testing of human samples were licensed and certified by the Clinical Laboratory Improvement Amendments. Samples were obtained at a single time point with the goal of testing subjects at 6–9 months after the estimated date of infection. Inflammatory protein levels in <15% of samples were below the limit of detection (0.6 pg/mL for TNF-α, IL-6, IL-1β, and IFN-γ; 0.1 μg/mL for CRP; and 10 ng/mL for D-dimer).
Statistical Analysis
Spearman's rank correlation coefficient was used to examine bivariate associations between inflammatory proteins and viral load set point and CD4+ T-cell count. Bonferroni correction was used to correct for multiple comparisons, but nominal unadjusted P values are presented because of the modest sample size and exploratory nature of the analysis. The Mann–Whitney test assessed differences in inflammatory protein levels for groups based on CD4+ T-cell counts above or below 500 cells/mm3. Log-rank (Kaplan–Meier) analyses summarized time-to-event data for the primary and secondary end points. Only subjects who had not reached the end point at the time of testing were included (47 subjects, for days to reach a CD4+ T-cell count of <500 cells/mm3; 72, for days to reach a CD4+ T-cell count of <350 cells/mm3; and 84, for days to initiation of ART). Cox proportional hazard ratios were use to estimate the univariate and adjusted risk of reaching predefined end points in groups divided into high and low categories on the basis of mean values (the median value was used for viral load). All P values are 2 sided, and P values of < .05 were considered statistically significant. Statistical analyses were conducted using Prism 5 (GraphPad, La Jolla, CA) and SAS v9.2 (SAS, Cary, NC).
RESULTS
To examine the role of inflammation during early HIV-1 infection, a retrospective analysis was conducted on the Boston Acute and Early HIV-1 Study cohort. All patients were enrolled within 6 months of HIV-1 infection and were followed longitudinally to record clinical data, CD4+ T-cell count, and HIV-1 load and to obtain blood samples. As our focus was early HIV-1 disease progression, we excluded subjects who received ART within the first 2 months after enrollment or who had <6 months of follow-up. Supplementary Table 1 shows the characteristics of our study cohort (n = 90 patients) and clinical data at the time of inflammatory protein testing. Plasma samples were tested for TNF-α, IFN-γ, IL-1β, IL-6, CRP, and D-dimer levels, and results were analyzed for association with viral load set point and time to clinical end points.
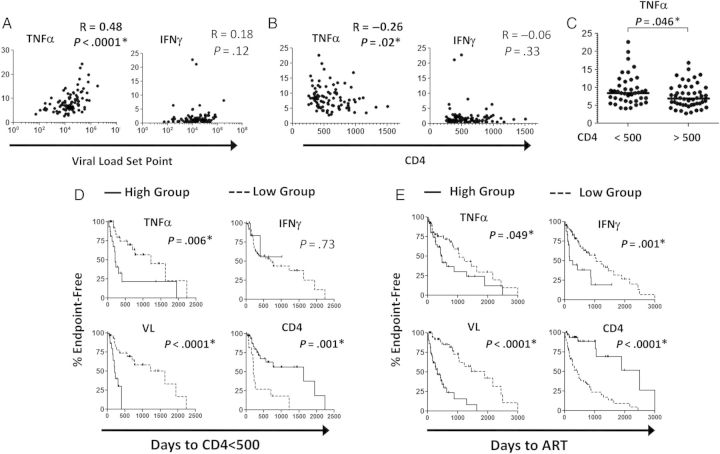
Among the inflammatory proteins tested, only TNF-α levels strongly correlated with viral load set point (R = 0.48, P < .0001; Figure 1A) and CD4+ T-cell count (R = −0.26, P = .02; Figure 1B) by Spearman rank correlation. Bonferroni correction for multiple comparisons confirmed the strong correlation between TNF-α and viral load set point (P < .001). TNF-α levels were also significantly higher in individuals with a concurrent CD4+ T-cell count <500 cells/mm3, compared with those with >500 cells/mm3 (P = .046, by the Mann–Whitney test; Figure 1C). Correlation analysis for IL-1β, IL-6, CRP, and D-dimer levels were nonsignificant, while CD4+ T-cell count was significantly inversely correlated to viral load set point (R = −0.42, P = .003; Supplementary Figure 1). Taken together, these data support the hypothesis that the TNF-α level is associated with early measures of HIV-1 disease severity, as manifested by viral load set point and CD4+ T-cell count.
Figure 1.
Association of tumor necrosis factor α (TNF-α) level with viral load set point, CD4+ T-cell count, and early human immunodeficiency virus type 1 (HIV-1) disease progression. A and B, TNF-α and interferon γ (IFN-γ) levels were measured in study subjects and compared to the viral load set point (A) and CD4+ T-cell count (B) at the time of testing, using Spearman rank correlation analysis. Nominal unadjusted 2-sided P values are presented. C, The TNF-α level was significantly higher (as determined by the Mann–Whitney test) in subjects with a CD4+ T-cell count of <500 cells/mm3. D and E, Log-rank analysis was used to determine the interval between testing and reaching the end points of a CD4+ T-cell count of <500 cells/mm3 (D) and initiation of antiretroviral therapy (ART; E) Subjects were categorized as having a high or low TNF-α level, IFN-γ level, or CD4+ T-cell count, by using the mean value as a cutoff; the median value was used as a cutoff for viral load due to the logarithmic distribution. A P value of <.05 is considered statistically significant.
We next investigated whether the plasma levels of inflammatory proteins were associated with clinical measures of early HIV-1 disease progression. Subjects were classified into high or low groups as described above. Log-rank analysis revealed that the group with high TNF-α levels (≥8.5 pg/mL) had significantly faster progression to a CD4+ T-cell count of <500 cells/mm3 (P = .006; Figure 1D) and earlier initiation of ART (P = .049; Figure 1E). The group with high TNF-α levels took a median of 208 days to reach a CD4+ T-cell count of <500 cells/mm3, compared with 1218 days in the group with low TNF-α levels. The median times to ART initiation were 439 and 1057 days in the high and low TNF-α groups respectively. A high IFN-γ level (≥2.0 pg/mL) was significantly associated with shorter time to initiation of ART only (P = .001; median, 167 vs 1057 days in the high and low groups, respectively). IL-1β, IL-6, CRP, and D-dimer levels did not show significant differences between the high and low groups (Supplementary Figure 1). Additional data from the log-rank analyses of the high and low groups, including the median times to reach end points, are presented in Supplementary Table 2. The time to reach a CD4+ T-cell count of <350 cells/mm3 was not significantly different between the high and low groups for any of the inflammatory proteins tested (data not shown). These results show that plasma TNF-α levels are highly associated with early HIV-1 disease progression, similar to associations found with viral load set point and CD4+ T-cell count.
To further investigate the risk associated with inflammation in early HIV-1 disease progression and determine the contribution of viral load and CD4+ T-cell count, we applied a multivariate Cox proportional hazard model. For the time to reach a CD4+ T-cell count of <500 cells/mm3, the ratio of the hazard for the group with high TNF-α levels to that for the group with low TNF-α levels was 3.1 (95% CI, 1.3–7.1; P < .01) in the univariate analysis, 1.9 (95% CI, .8–4.7; P = .15) when adjusted for concurrent viral load, and 5.1 (95% CI, 1.9–13.5; P = .001) when adjusted for concurrent CD4+ T-cell count (Table 1). The group with high TNF-α levels had an increased risk for the time to ART initiation in univariate analysis (hazard ratio [HR] 1.8; 95% CI, 1.0–3.2), although the difference was not statistically significant (P = .053). The group with high IFN-γ levels had an increased hazard only for the time to ART initiation (HR, 2.9; 95% CI, 1.5–5.5; P = .002); the increase remained statistically significant after adjustment for viral load (HR, 2.1; 95% CI, 1.1–4.1; P = .03) or CD4+ T-cell count (HR, 1.9; 95% CI, 1.0–3.7; P = .05). High viral load and low CD4+ T-cell count yielded an increased risk of clinical progression, as expected. Because of the modest number of events, Cox models separately adjusted for log10 HIV-1 RNA load and CD4+ T-cell count but not both. Cox analysis for days to reach a CD4+ T-cell count of <350 cells/mm3 did not show significant HRs for any of the inflammatory proteins tested (data not shown). Thus, these studies demonstrate that patients with HIV-1 infection who have high plasma levels of TNF-α have an increased risk of reaching a CD4+ T-cell count of <500 cell/mm3, which is dependent on viral load but independent of CD4+ T-cell count at the time of testing.
Table 1.
Cox Proportional Hazard Ratios (HRs) for Inflammatory Proteins in Early Human Immunodeficiency Virus Type 1 (HIV-1) Infection
| End Point, Factor | Multivariate Analyses Adjusted for |
|||||
|---|---|---|---|---|---|---|
| Univariate Analyses |
Viral Load |
CD4+ T-Cell Count |
||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Time to CD4+ T-cell count <500 cells/mm3 (n = 47a) | ||||||
| TNF-α level | 3.1 (1.3–7.1) | .008 | 1.9 (.8–4.7) | .15 | 5.1 (1.9–13.5) | .001 |
| IL-6 level | 0.8 (.3–2.4) | .70 | NDb | NDb | ||
| CRP level | 0.5 (.1–1.5) | .20 | NDb | NDb | ||
| D-dimer level | 1.0 (.4–2.3) | .91 | NDb | NDb | ||
| IL-1β level | 1.0 (.4–2.4) | .95 | NDb | NDb | ||
| IFN-γ level | 0.8 (.2–3.4) | .73 | NDb | NDb | ||
| Viral load | 5.2 (1.9–14.4) | .001 | NA | 5.0 (1.7–14.1) | .003 | |
| CD4+ T-cell count | 3.9 (1.6–9.2) | .002 | 3.1 (1.3–7.5) | .01 | NA | |
| Time to ART initiation (n = 84a) | ||||||
| TNF-α level | 1.8 (1.0–3.2) | .053 | NDb | NDb | ||
| IL-6 level | 1.4 (.7–2.9) | .33 | NDb | NDb | ||
| CRP level | 1.1 (.5–2.4) | .78 | NDb | NDb | ||
| D-dimer level | 1.4 (.8–2.5) | .31 | NDb | NDb | ||
| IL-1β level | 0.6 (.3–1.2) | .15 | NDb | NDb | ||
| IFN-γ level | 2.9 (1.5–5.5) | .002 | 2.1 (1.1–4.1) | .03 | 1.9 (1.0–3.7) | .05 |
| Viral load | 6.1 (3.0–12.3) | <.001 | NA | 3.4 (1.7–7.1) | <.001 | |
| CD4+ T-cell count | 6.2 (2.6–14.8) | <.001 | 2.8 (1.1–7.1) | .03 | NA | |
Multivariate analyses adjusted for 1 log10 HIV-1 load or 100-cell increments in CD4+ T-cell count. A P value of <.05 is considered statistically significant.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CRP, C-reactive protein; IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-6, interleukin 6; NA, not applicable; TNF-α, tumor necrosis factor α.
a Subjects who reached the end point at or before inflammatory protein testing were excluded from the analysis.
b Not done (ND) because of the lack of statistical significance in the univariate model.
DISCUSSION
The central role of inflammation in HIV-1 disease pathogenesis and immune dysfunction is increasingly being recognized [11, 12]. While inflammation has been extensively studied in chronic HIV-1 infection, here we show that a high level of TNF-α but not other well-described inflammatory proteins was associated with a decreased time to reach a CD4+ T-cell count of <500 cells/mm3 and to initiation of ART during early HIV-1 infection. This association of high TNF-α levels with early HIV-1 disease progression was dependent on viral load but independent of concurrent CD4+ T-cell count. Thus, TNF-α appears to be an important mediator of inflammation driven by high levels of viral replication, which is linked to early HIV-1 disease progression. Interestingly, CD4+ T-cell count, which is the primary benchmark used worldwide to recommend ART initiation, had only a weak association with TNF-α level and did not reduce the risk for early disease progression seen in the high TNF-α group after adjustment for this parameter.
Previous studies have characterized inflammation during early HIV-1 infection and attempted to determine the relationship with disease outcome. Nearly two decades ago, Rizzardi et al demonstrated increased TNF-α levels in HIV-1–infected individuals who went on to develop an AIDS-related outcome, albeit with a much smaller sample size [13]. A recent study showed that the IP-10 level during acute HIV-1 infection was correlated with CD4+ T-cell count and T-cell activation measured six months later (interestingly, the TNF-α level was not associated) [14]. However, these studies did not examine associations with the time to HIV-1 disease progression or the contribution of viral replication to inflammation.
Given the importance of viral load in TNF-α–mediated inflammation during early HIV-1 infection, additional studies are required to understand the mechanism leading to increased TNF-α production, as well as to determine any long-term impact on immune dysfunction. Chronically high exposure to TNF-α is likely to have negative effects in the individual, but it is unclear whether this is reversible or whether it is linked to late inflammation associated with elevated IL-6 and D-dimer levels. Some insight into this is provided by a recent study by Tabb et al, who used TNF-α inhibitors during simian immunodeficiency virus infection in rhesus macaques. This study found that blocking TNF-α did not affect viral load or T-cell activation but reduced inflammation at the lymphoid tissue level [15]. The findings from our study further support the current body of literature, which suggests that inflammation related to high viral replication plays an important role in HIV-1–related pathology and that strategies to counteract inflammation may help reduce or postpone disease progression.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the patients who participated in this study and the clinical staff, for their assistance; and Musie Ghebremichael, Eileen Scully, and Keith Rands, for helpful discussions.
S. A. V. and M. Altfeld designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript. C. K. and M. S. assisted with sample testing and data interpretation. J. R. enrolled the study subjects and managed the clinical database. M. Amero and S. B. assisted with obtaining clinical data and patient samples. T. M. A. and E. S. R. enrolled study subjects and assisted with data interpretation. R. J. B. assisted with data interpretation, statistical analysis, and editing the manuscript. All authors read and approved the final manuscript.
Financial support. This work was supported by the National Institutes of Health (grants P01 AI074415 [to M. Altfeld and R. J. B.] and T32 AI07387-23 and P30 AI060354-10 [to S. A. V.]), the Bill and Melinda Gates Foundation, the Doris Duke Charitable Foundation (grant 208682), and the Philip T. and Susan Ragon Foundation.
Potential conflicts of interest. The authors state that they have no conflicts of interest or financial disclosures.All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–36. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating SM, Jacobs ES, Norris PJ. Soluble mediators of inflammation in HIV and their implications for therapeutics and vaccine development. Cytokine Growth Factor Rev. 2012;23:193–206. doi: 10.1016/j.cytogfr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses. 2006;22:757–62. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassutto S, Maghsoudi K, Johnston MN, et al. Longitudinal analysis of clinical markers following antiretroviral therapy initiated during acute or early HIV type 1 infection. Clin Infect Dis. 2006;42:1024–31. doi: 10.1086/500410. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Boer RJ, Borghans JA. Immune Activation and Collateral Damage in AIDS Pathogenesis. Front Immunol. 2013;4:298. doi: 10.3389/fimmu.2013.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzardi GP, Barcellini W, Tambussi G, et al. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS. 1996;10:F45–50. doi: 10.1097/00002030-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Liovat AS, Rey-Cuille MA, Lecuroux C, et al. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One. 2012;7:e46143. doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabb B, Morcock DR, Trubey CM, et al. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis. 2013;207:880–92. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.