Abstract
Background
The role of FOXP3+ regulatory T cells in the prevention against sensitization and allergy development is controversial.
Objective
We followed 65 newborn Swedish children from farming and non-farming families from birth to 3 years of age and investigated the relation between CD4+ T cell subsets in blood samples and development of sensitization and allergic disease.
Methods
The proportions of FOXP3+CD25high, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ within the CD4+ T cell population were examined by flow cytometry of blood samples at several time-points. Mononuclear cells were isolated from blood and stimulated with birch allergen, ovalbumin or the mitogen PHA, and the levels of IL-1β, IL-6, TNF, IFN-γ, IL-5 and IL-13 were measured. A clinical evaluation regarding the presence of allergen-specific IgE and allergy was performed at 18 and 36 months of age.
Results
Multivariate discriminant analysis revealed that children who were sensitized at 18 or 36 months of age had higher proportions of FOXP3+CD25high T cells at birth and at 3 days of life than children who remained non-sensitized, whereas allergy was unrelated to the neonatal proportions of these cells. The proportions of CTLA-4+CD25+ T cells were unrelated to both sensitization and allergy. The association between higher proportions of FOXP3+CD25high T cells and sensitization persisted after exclusion of farmer's children. Finally, a farming environment was associated with lower proportions of FOXP3+CD25high T cells in early infancy and to a more prominent T cell memory conversion and cytokine production.
Conclusion & Clinical Relevance
Our results indicate that high proportions of FOXP3+CD25high T cells in neonates are not protective against later sensitization or development of allergy.
Keywords: allergic sensitization, children, farm, FOXP3+CD25high T cells, neonates, T cell development
Introduction
Production of IgE antibodies to allergens is termed sensitization 1. In some individuals, allergen-specific IgE antibodies can trigger inflammation upon renewed exposure to allergens, giving rise to immune-mediated hypersensitivity, that is, allergy. Th2 cells produce cytokines such as IL-4, IL-5 and IL-13 that support the production of allergen-specific IgE and recruitment of eosinophils 2. Although sensitization can occur without symptoms of allergic diseases, children who are sensitized to any allergen at 18 months of age without allergic symptoms are more likely than others to develop symptoms of wheezing, asthma and rhinitis at 5 years of age 3.
Regulatory T cells (Tregs) play a central role in the maintenance of self-tolerance and immune homoeostasis 4. Human Tregs from peripheral blood and thymus suppress proliferation and cytokine production of other T cells in response to both self-antigens and allergens 5–7. Tregs are found within the CD4+ T cell population and express CD25 and the transcription factor FOXP3 8. Mutations in the FOXP3 gene lead to a deficiency in Tregs and to the syndrome X-linked autoimmunity-allergic dysregulation, characterized by organ-specific autoimmunity, enterocolitis with food allergy and severe dermatitis 9,10. The fraction of FOXP3+ cells is higher within the CD25high (approximately the top 2%) compared with the total CD25+ T cell subset 11. As not only Tregs but also newly activated CD4+ T cells express CD25 and FOXP3 12, analysis of the FOXP3+CD25high T cell subset results in a lower contamination of activated non-regulatory T cells 13.
Significant efforts have been made to identify the roles played by Tregs and immunomodulatory cytokines in the development of allergy. However, the majority of studies demonstrating altered or impaired immunomodulatory T cell phenotypes in allergic individuals have focused on adults or on children with established allergy. Some studies have correlated the proportion of Tregs at birth and in infants with subsequent onset of sensitization and/or allergic disease later in childhood, but with inconsistent results. Thus, certain studies demonstrate lower numbers 14 or poor function 15 of putative Tregs at birth in children who later become sensitized and/or allergic. Other studies show that the proportions of FOXP3+ Tregs at birth do not differ significantly between children with subsequent sensitization or allergic disease 16,17. However, in one of the latter studies, established sensitization and/or allergic disease were associated with higher proportions of FOXP3+ T cells 16. Thus, it still remains unclear whether alterations in the proportions of Tregs or in their regulatory capacity precede development of sensitization and allergic disease.
Epidemiological studies strongly suggest that reduced microbial exposure early in life leads to the development of allergy later in childhood 18–20. Growing up on a farm exerts a strong protective effect against development of both sensitization and, even more strongly, allergic disease 20,21. However, whether the post-natal development of the adaptive immune system differs between farmers' and non-farmers' children is currently unknown.
In the FARMFLORA study, we have prospectively followed 65 Swedish children from birth to 3 years of age, half of whom lived on small family-owned dairy farms and half in the same rural area, but not on farms. We examined the proportions of FOXP3- or CTLA-4-expressing T cells as well as T cell activation, memory conversion and homing markers, and whether the pattern of T cell subsets and activity status was related to the development of sensitization and allergic disease later in childhood. By the use of multivariate discriminant analysis, we found a positive association between high proportions of FOXP3+CD25high T cells in cord blood and in early infancy and sensitization at both 18 and 36 months of age. We also found that higher proportions of these cells early in infancy were associated with a non-farming environment. Although the prevalence of allergy was significantly lower among children who grew up on small dairy farms, the farming environment was not a confounding factor for the associations between higher proportions of FOXP3+ T cells and sensitization in this study.
Materials and methods
Subjects and collection of blood samples
In total, 65 healthy Swedish infants born at term (≥ 38 gestational weeks) in rural areas in south-west of Sweden were included in the prospective FARMFLORA study. Twenty-eight of the children were raised on small dairy farms, while 37 lived on the countryside, but not on a farm. Blood samples were obtained from the umbilical cord at birth and peripheral blood was sampled at 3–5 days, at 1, 4, 18 and 36 months of age. Informed consent was obtained from the parents, and the study protocol was approved by the Human Research Ethics Committee of the Medical Faculty, University of Gothenburg, Sweden (reference number 363-05).
Clinical and laboratory examinations for allergy diagnoses
The children were examined by a paediatrician at 18 and 36 months of age, and between follow-ups if they developed symptoms suggestive of allergic disease. Venous blood was collected for total IgE and the presence of specific IgE against food (milk, egg, soy, fish, wheat and peanut, Phadia/Pharmacia Diagnostics, Uppsala, Sweden) and inhalant allergens (birch, timothy grass, mugwort, cat, dog, horse, and house dust mite Phadia/Pharmacia). Positive samples were further analysed for specific IgE against birch, timothy, mugwort, dog, cat, horse, house dust mite, cow's milk, hen's egg, fish, wheat, soy and peanut (Immunocap®; Phadia/Pharmacia Diagnostics). An allergen-specific IgE level of ≥ 0.35 kU/L was considered positive. In the evaluation of children who were referred to the study paediatrician before 18 months of age because of symptoms, allergen-specific IgE-tests (Immunocap) or skin prick tests were performed guided by symptoms. Skin prick tests were performed in accordance with European guidelines using standard allergen extracts (Soluprick SQ; ALK-Abello AS, Hørsholm, Denmark), allergen diluents as the negative control and histamine (10 mg/mL) as positive control.
Diagnostic groups
Based on clinical examination by the paediatrician and the results of the laboratory tests, the following diagnostic groups were defined: Eczema, diagnosed according to Williams' criteria 22. Eczema at 18 months denoted diagnosis at any time before or at 18 months, while eczema at 36 months required symptoms to be present after 24 months of age; Asthma, persistent wheezing for ≥ 4 weeks or ≥ 3 episodes of wheezing in the first 18 months of life in combination with other manifestations of allergy (eczema, rhinoconjunctivitis or food allergy) or with wheeze/breathing problems in between colds. For asthma at 36 months, ≥ 1 wheezing episode should have occurred after 24 months of age, and response to inhaled glucocorticoids or leukotriene antagonists was included among the minor criteria; Food allergy, an immediate- or late-onset reaction after ingestion of the specific food, followed by a clear and prompt clinical improvement once the food allergen was eliminated. The diagnosis was supported by open food challenge tests, and/or a positive specific allergy test (specific IgE ≥ 0.35 kU/L or skin prick test weal ≥ 3 mm), and/or eosinophilic inflammation in mucosal gastrointestinal biopsies, and/or multi-organ reactions; Allergic rhinoconjunctivitis, symptoms in the eyes and/or nose upon exposure to pollens or animal dander, together with a positive allergen-specific IgE test directed against a corresponding allergen.
Flow cytometry
Phenotypic characterization of CD4+ T lymphocytes was performed as described in detail elsewhere 23. The following anti-human monoclonal antibodies were used on whole blood: APC-conjugated anti-CD25 (clone 2A3; BD Biosciences, Erembodegem, Belgium) and HLA-DR (clone G46-6, BD Bioscience); FITC-conjugated anti-CD49d (clone 44H6; Serotec, UK); PerCP-conjugated anti-CD4 (clone SK3; BD Biosciences); and PE-conjugated anti-CD45RO (clone UCHL-1; BD Biosciences), anti-β7-integrin (clone FIB504; BD Biosciences) and anti-CCR4 (clone IG1; BD Biosciences). For intracellular FOXP3 staining, the PE anti-human FOXP3 staining set was used (clone PCH101; eBioscience, San Diego, CA, USA), whereas the Cytofix/Cytoperm kit (BD Biosciences) was used for biotin-conjugated CTLA-4 (clone BNI3; BD PharMingen, San Diego, CA, USA), followed by PE-conjugated streptavidin (BD Biosciences). This staining method allows detection of both intra- and extracellular CTLA-4. All isotype controls were purchased from BD Biosciences, except for the isotype control for FOXP3, which was purchased from eBioscience. The gates for expression of FOXP3 or CTLA-4 were set based on the expression of these respective markers within the CD25high and CD25+ cell subsets, and the lack of expression in within the CD25− subset 11. The proportion of FOXP3+CD25high cells within the CD4+ T cell population was calculated by multiplying the proportion of CD25high cells within the CD4+ T cell population (˜2%) with the percentage of FOXP3+ of CD25high cells. FOXP3, CTLA-4 and CD45RO were analysed in blood obtained at birth, 3–5 days, 1, 4, 18 and 36 months, whereas HLA-DR, α4β7 and CCR4 were analysed at birth and 4, 18 and 36 months of age. Samples were run in a FACSCalibur (BD Biosciences) equipped with CellQuest Pro software or in a FACSCanto II (BD Bioscience) equipped with FACSDiva software and analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Cell culture and cytokine determinations
Mononuclear cells isolated from blood samples obtained at 4, 18 and 36 months of age were stimulated with 5 μg/mL phytohaemagglutinin (PHA) (Roche, Basel, Switzerland) for 24 hours. Cells obtained at 36 months of age were stimulated with 100 μg/mL birch allergen extract (ALK ABELLÓ, Hørsholm, Denmark) or 200 μg/mL ovalbumin (OVA) (Sigma Aldrich, St Louis, MO, USA) for 6 days. Cells were cultured at 1x106 cells/mL in AIM-V media (Gibco, Life technologies, Paisley, UK), supplemented with 40 μM mercaptoethanol (Merck Millipore, Darmstadt, Germany) in U-bottomed 96-well plates and kept in 5% CO2 at 37°C. Concentrations of IL-1β, IL-6, TNF, IFN-γ, IL-5 and IL-13 were measured in the supernatant by FlowCytomix (eBioscience, Vienna, Austria), and the levels of IFN-γ and IL-13 were confirmed by ELISA, as described in detail previously 24. All cytokine concentrations are shown in Table S1. IL-1β, IFN-γ and IL-5 at 4 months of age were excluded due to high number of non-responders.
Statistical analysis
The associations between the proportions of FOXP3+CD25high, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ cells within the CD4+ T cell population, and cytokine production (X-variables), and clinical diagnosis of allergy or sensitization (Y-variables) were investigated in multivariate factor analyses (SIMCA software, version 13.0; Umetrics, Umeå, Sweden). Orthogonal projections to latent structures discriminant analyses (OPLS-DA) were implemented to examine whether classes of observations, that is, sensitized compared with non-sensitized children, allergic compared with non-allergic children and farmers' compared with non-farmers' children, could be discriminated based on the totality of X-variables. The quality of the analyses was based on the parameters R2, that is, how well the variation of the variables is explained by the model, and Q2, that is, how well a variable can be predicted by the model. In the OPLS-DA loadings column plots, the importance of each X-variable to Y is represented by column bars. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The final OPLS-DA loadings column plots in the result section are models based on X-variables with variable influence of projection (VIP values) values ≥ 0.8, ≥ 0.9 or ≥ 1.0 (described in the figure legends). VIP values can be used to discriminate between important and unimportant predictors for the overall model. To avoid mass significance, univariate analyses were performed exclusively on the X-variables that contributed most to the respective models. Univariate data analyses were performed with Mann–Whitney U-test or chi-square test (GraphPad Prism; GraphPad Software, San Diego, CA, USA) as described in the figure legends. A P-value ≤ 0.05 was considered significant (*P ≤ 0.05; ** P ≤ 0.01; and ***P ≤ 0.001).
Results
Allergy and sensitization
By the age of 18 and 36 months, 23% and 17% of the children, respectively, were diagnosed as being allergic (Table1). Sensitization occurred in 15% and 25% of the children at these time-points, respectively (Table1). For further analyses, we compared those who were allergic at 18 months of age (n = 15) with those who were not (n = 49). Children who were allergic at 36 months of age (n = 11) were compared with those who were not allergic at either 18 or 36 months of age (n = 44). For this analysis, eight children who were allergic at 18 but not at 36 months of age were excluded, as we considered that they could be included neither in the allergic nor in the non-allergic group. One child who did not undergo clinical examination at 18 months of age was, hence, excluded from both the 18 and 36 month analyses. Sensitized and non-sensitized children were compared in the same fashion. Sensitization at both 18 and 36 months of age was more prevalent among allergic relative to non-allergic children at 36 months (P = 0.03 and P = 0.05, respectively). Total IgE levels did not differ between allergic and non-allergic children either at 18 (32 kU/L vs. 19 kU/L, P = 0.68) or 36 (130 kU/L vs. 20 kU/L, P = 0.07) months of age. A double parental history of allergy was more common among children diagnosed with allergy at 18 months of age than among non-allergic children (Table2). Furthermore, boys were overrepresented among those children who were allergic by 36 months of age.
Table 1.
Clinical diagnosis of allergic sensitization and allergic disease of children at 18 and 36 months of age
| 18 months
(n = 64) |
36 months
(n = 63) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Any allergy* | 15 | 23 | 11 | 17 |
| Food allergy | 2 | 3 | 2 | 3 |
| Eczema | 13 | 20 | 7 | 11 |
| Allergic rhinoconjunctivitis | 1 | 2 | 1 | 2 |
| Asthma | 5 | 8 | 4 | 6 |
| Sensitization† | 9 | 15‡ | 14 | 25§ |
One or more of the following diagnoses: eczema, asthma, food allergy or allergic rhinoconjunctivitis.
Screening for allergen-specific IgE, that is, 6-mix food test and Phadiatop, followed by analysis for specific IgE against cow's milk, hen's egg, fish, wheat, soy, peanut, birch, timothy, mugwort, dog, cat, horse and house dust mite.
n = 62 (blood samples not available for all 64 children).
n = 56 (blood samples not available for all 63 children).
Table 2.
Demographic data
| Number of children (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitized children |
Allergic children |
||||||||||||
| At 18 months |
At 36 months |
At 18 months |
At 36 months |
||||||||||
| All n = 65 |
No n = 53 |
Yes n = 9 |
P-value* | No n = 39 |
Yes n = 14 |
P-value* | No n = 49 |
Yes n = 15 |
P-value* | No n = 44 |
Yes n = 11 |
P-value* | |
| Boys | 33 (51) | 27 (51) | 5 (56) | 1.0 | 21 (54) | 9 (64) | 0.55 | 24 (49) | 9 (60) | 0.56 | 22 (50) | 9 (82) | 0.03 |
| Single parental history of allergy† | 22 (34) | 18 (34) | 2 (22) | 0.71 | 14 (36) | 5 (36) | 1.0 | 17 (35) | 4 (27) | 0.76 | 14 (32) | 5 (45) | 0.48 |
| Double parental history of allergy† | 5 (8) | 5 (9) | 0 (0) | 1.0 | 4 (10) | 1 (7) | 1.0 | 1 (2) | 4 (27) | 0.01 | 1 (2) | 2 (18) | 0.1 |
| Sibling(s) | 35 (54) | 30 (57) | 4 (44) | 0.72 | 22 (56) | 7 (50) | 0.76 | 28 (57) | 7 (47) | 0.56 | 23 (52) | 6 (55) | 1.0 |
| Caesarean section | 10 (15) | 7 (13) | 3 (33) | 0.15 | 5 (13) | 5 (36) | 0.11 | 6 (12) | 4 (27) | 0.23 | 5 (11) | 4 (36) | 0.07 |
Statistical difference between non-sensitized and sensitized children or between non-allergic and allergic children (Fisher's exact test or Mann–Whitney U-test). Bold: P ≤ 0.05 was regarded as significant (*P ≤ 0.05 and **P ≤ 0.01).
Maternal or paternal history of doctor-diagnosed asthma, allergic rhinoconjunctivitis or eczema.
High proportions of neonatal FOXP3+CD25high T cells are positively associated with sensitization later in childhood
Using multivariate discriminant analysis by OPLS (orthogonal projections to latent structures), we investigated sensitization at 18 or 36 months of age in relation to proportions of CD4+ T cell subsets (FOXP3+CD25high, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ and α4β7+) at birth, 3–5 days of age and at 1, 4, 18 and 36 months of age, as well as cytokine production (IL-1β, TNF, IL-6, IFN-γ, IL-5 and IL-13) by peripheral blood mononuclear cells after stimulation with PHA (at 4, 18 and 36 months of age), birch allergen extract or ovalbumin (at 36 months of age). For sensitization at 18 months of age, immune parameters measured at 36 months were not included in the analysis. The OPLS-DA scatter plots, in Figs1a and b, indicated that children who were sensitized (filled circles) and non-sensitized (open circles) at 18 or 36 months of age, respectively, could be separated based on the T cell variables described above.
Figure 1.

(a and b) OPLS discriminant analysis (OPLS-DA) score scatter plot displaying the separation of sensitized, that is, specific IgE to food and/or inhalant allergens, (filled circles) and non-sensitized (open circles) children at (a) 18 and (b) 36 months of age based on X-variables, including CD4+ T cells that are FOXP3+CD25high, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ at birth, 3–5 days, and at 1, 4, 18, 36, months of age. Measurements of PHA-induced cytokine production by mononuclear cells at 4, 18 and 36 months and OVA- and birch allergen extract-induced cytokine production at 36 months of age were also included in the analyses. For sensitization at 18 months, immune parameters measured at 36 months were not included. R2Y indicates how well the variation of Y is explained, while Q2 indicates how well Y can be predicted. (c and d) OPLS-DA loadings column plots depicting the associations between the X-variables described above and sensitization at (c) 18 and (d) 36 months of age. X-variables with bars projected in the same direction as sensitization are positively associated, whereas bars in the opposite direction are inversely related to sensitization at the respective ages. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The OPLS loadings column plots are based on X-variables with VIP values (c) ≥ 0.8 and (d) ≥ 0.9. R2Y: (c) = 0.28, (d) = 0.37. Q2: (c) = 0.23, (d) = 0.24.
The T cell variables that displayed the strongest association, positive or negative, with sensitization at 18 or 36 months of age are identified in the OPLS-DA loadings column plots shown in Figs1c and d. T cell variables positioned in the same direction as the bar representing sensitization are positively associated, whereas parameters represented by a bar in the opposite direction are inversely related to sensitization. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The final OPLS-DA loadings column plots are based on parameters with VIP values ≥ 0.8 in Fig.1c and ≥ 0.9 in Fig.1d. The full VIP plots are shown in Figs S1a and b. As shown in Figs1c and d, allergic sensitization at 18 or 36 months of age was positively associated with higher proportions of FOXP3+CD25high T cells at both birth and at 3 days of life. In contrast, sensitization was related to lower levels of PHA-induced cytokine production by mononuclear cells (sensitization at 18 months: TNF, IL-1β, IL-6 and IFN-γ; sensitization at 36 months: IFN-γ and TNF). Taken together, these results imply that sensitization in the first 3 years of life is related to higher proportions of neonatal putative Tregs and a lower cytokine-producing capacity by mononuclear cells later in childhood.
Sensitized children have significantly higher proportions of FOXP3+CD25high T cells at birth and in early infancy than non-sensitized children
Univariate analyses demonstrate that children who were sensitized at 18 or 36 months of age had significantly higher proportions of FOXP3+CD25high cells within the CD4+ T cell population at birth and at 3 days of life than non-sensitized children (Figs2a and b). Despite the fact that Tregs are considered to express the immunoregulatory molecule CTLA-4 25,26, the proportions of CTLA-4+CD25+ T cells were unrelated to sensitization in both multivariate (Figs1c and d) and univariate analyses (Figs2c and d). The gating strategies for FOXP3+CD25high and CTLA-4+CD25+ expression within the CD4+ T cell population are demonstrated in Fig.2e.
Figure 2.
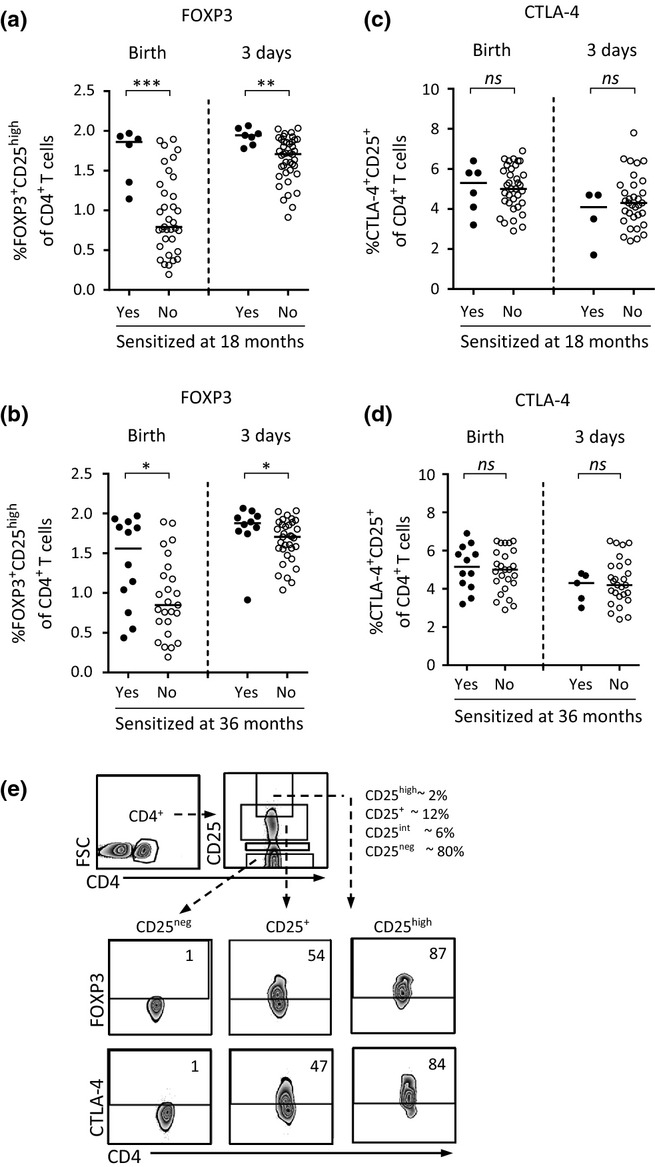
(a and b) The proportions of FOXP3+CD25high cells within the CD4+ T cell population at birth and at 3–5 days in children who were sensitized, that is, specific IgE to food and/or inhalant allergens, or non-sensitized at (a) 18 or (b) 36 months of age. (c and d) The proportions of CTLA-4+CD25+ cells within the CD4+ T cell population at birth and at 3–5 days in children who were sensitized or non-sensitized at (c) 18 or (d) 36 months of age. Each dot represents an individual, and horizontal bars indicate median value. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U-test. P ≤ 0.05 was regarded as significant (*P ≤ 0.05; ** P ≤ 0.01; and *** P ≤ 0.001). (e) Gating strategies for FOXP3 or CTLA-4 expression within the various CD25+ T cell populations in cord blood. Numbers represent the percentage of cells within the gate.
The association between sensitization and lower PHA-induced cytokine production observed in multivariate analyses (Figs1c and d) was not supported by univariate analyses. However, there was a trend that children who were sensitized at 18 months of age produced lower levels of IL-1β at 18 months (P = 0.07), whereas children who were sensitized at 36 months of age tended to produce lower levels of and IFN-γ at 18 months (P = 0.06). Taken together, these results show that children who were sensitized at 18 or 36 months of age had significantly higher proportions of FOXP3+CD25high cells within the CD4+ T cell population at birth and at 3 days of life than children who remained non-sensitized.
Furthermore, as others have used the FOXP3+CD25+ phenotype to define Tregs, we also performed corresponding analyses with the use of this cell subset. These multivariate analyses displayed a similar association pattern with subsequent development of allergic sensitization as in Figs.1a and b (Figure S2A and B). However, in contrast to FOXP3+CD25high T cells (Fig.2), univariate analyses showed that the proportions of FOXP3+CD25+ were significantly higher only at birth, and not at 3 days of life, among children with allergic sensitization at 18, but not 36 months of age compared with non-sensitized children (Figures S2c and d). As the proportions of neonatal FOXP3+CD25high but not FOXP3+CD25+ differed significantly between children who later developed allergic sensitization, this may indicate that the latter cell subset could be contaminated by activated non-regulatory T cells.
Allergy in relation to T cell maturation progress
Although allergy is linked to sensitization, children may be allergic but not sensitized, as well as sensitized but not allergic (Table1). Hence, we examined how the T cell variables described above were related to allergic disease at 18 and 36 months of age. The OPLS-DA loadings column plot in Fig.3a is based on parameters with VIP values ≥ 0.9. Being allergic at 36 months of age was associated with mononuclear cells with a higher capacity to produce the Th2-related cytokines IL-5 and IL-13 at both 18 and 36 months of life. However, no T cell variables were inversely related to allergic disease. Univariate analyses confirmed that allergic children produced significantly higher levels of IL-5 upon PHA stimulation at 36, but not at 18 months of age (Fig.3b). A clinical diagnosis of allergy at 18 months of age did not display any specific association patterns with the T cell variables (data not shown). These results suggest that, in contrast to sensitization, a clinical diagnosis of allergy is not associated with higher proportions of neonatal FOXP3+CD25high putative Tregs within the CD4+ T cell population. Moreover, as one could expect, being allergic is related to a higher capacity to produce the Th2-related cytokine IL-5.
Figure 3.
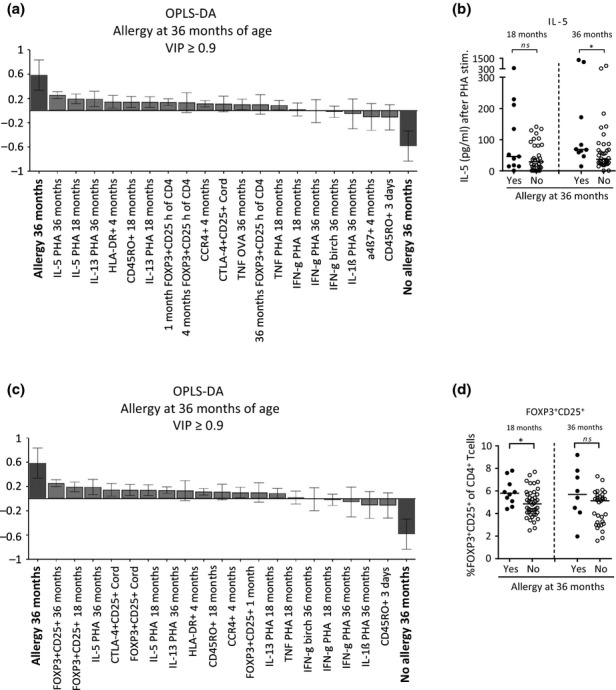
(a and c) OPLS-DA loadings column plot depicting the associations between allergic disease at 36 months, that is, children diagnosed with eczema, asthma, food allergy and/or allergic rhinoconjunctivitis, while specific IgE was not a criterion for a clinical diagnosis of allergy, and X-variables, including CD4+ T cells that are (a) FOXP3+CD25high or (c) FOXP3+CD25+, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ at birth, 3–5 days, and 1, 4, 18 and 36 months of age. Measurements of PHA-induced cytokine production by mononuclear cells at 4, 18 and 36 months and OVA- and birch allergen extract-induced cytokine production at 36 months of age were also included in the analyses. X-variables with bars projected in the same direction as allergy are positively associated, whereas parameters in the opposite direction are inversely related to allergy at this age. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The OPLS-DA loadings column plots are based on X-variables with VIP-values ≥ 0.9. R2Y indicates how well the variation of Y is explained, while Q2 indicates how well Y can be predicted. R2Y: (a) = 0.33, (c) = 0.32. Q2: (a) = 0.18, (c) = 0.21. (b) The concentration of PHA-induced production of IL-5 (pg/mL) by mononuclear cells from children who were allergic or not at 36 months of age. (d) The proportions of FOXP3+CD25+ cells within the CD4+ T cell population at 18 and 36 months of age in children who were allergic or not at 36 months of age. Each dot represents an individual, and horizontal bars indicate median value. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U-test. P ≤ 0.05 was regarded as significant (*P ≤ 0.05).
Interestingly, corresponding analyses of the proportions of FOXP3+CD25+ cells displayed that a clinical diagnosis of allergy at 36 months of age was positively associated with high proportions of FOXP3+CD25+ cells within the CD4+ T cell population at both 18 and 36 months of age as well as at birth (Fig.3c). Univariate analyses showed that children with a clinical diagnosis of allergy at 36 months of age had significantly higher proportions of FOXP3+CD25+ cells within the CD4+ T cell population at 18 months of age, but not at any other time-point, compared with non-allergic children (Fig.3d). The association between high proportions of FOXP3+CD25+ cells and allergy just before the onset of or during disease suggests that the FOXP3+CD25+ cell subset might contain activated non-regulatory T cells as a consequence of the allergic inflammation.
The dairy farm environment is not a confounding factor for the associations between high proportions of neonatal FOXP3+CD25high cells and sensitization later in childhood
Several independent studies have shown that the prevalence of allergy is significantly lower among children raised in a farming environment 19,27. In the present cohort, we found a significantly lower prevalence of allergic disease among children growing up on dairy farms (7% at 18 months, 4% at 36 months) than among the children growing up on the countryside in the same area, but not on farms (35% at 18 months, P = 0.02; 32% at 36 months, P = 0.02). However, there was no difference regarding the prevalence of sensitization between these two groups (11% vs. 17% at 18 months, P = 0.7; 27% vs. 26% at 36 months, P = 1.0). Thus, farming environment was not likely to be a confounding factor for the positive associations observed between high proportions of FOXP3+CD25high cells within the CD4+ T cell population at birth and at 3 days of life and subsequent sensitization. Hence, after exclusion of farmers' children from the univariate analyses shown in Figs2a and b, the same patterns were observed regarding higher proportions of FOXP3+CD25high T cells at birth as well as at 3 days of life and sensitization later in childhood (Fig.4a and b). Indeed, children who were sensitized at 18 months of age had significantly higher proportions of FOXP3+CD25high T cells at birth and at 3 days of life than children who did not become sensitized (Fig.4a). Although not statistically significant, this pattern was also observed for children sensitized at 36 months of age (Fig.4b). Further, exclusion of farmers' children from the analysis did not alter the outcome that children with allergic disease at 36 months of age produced significantly higher levels of PHA-induced IL-5 at 36 months of age than non-allergic children (Fig.4c). Taken together, these results indicate that farming environment per se is not likely to be a confounding factor for the associations observed between high proportions of FOXP3+CD25high T cells at birth and at 3 days of life and sensitization later in childhood.
Figure 4.
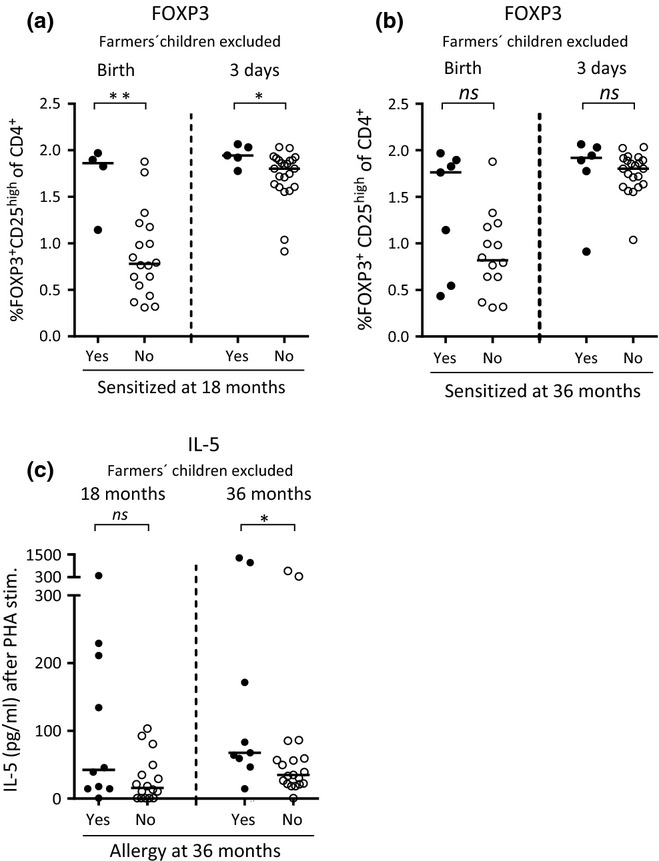
(a and b) The proportion of FOXP3+CD25high cells within the CD4+ T cell population at birth and at 3 days of age among non-farmers' children who were sensitized, that is, specific IgE to food and/or inhalant allergens, or non-sensitized at (a) 18 and (b) 36 months of age. (c) The concentration of PHA-induced production of IL-5 (pg/mL) by mononuclear cells from non-farmers' children who were allergic, that is, children diagnosed with eczema, asthma, food allergy and/or allergic rhinoconjunctivitis, while specific IgE was not a criterion for a clinical diagnosis of allergy, or not at 36 months of age. Each dot represents an individual, and horizontal bars indicate median values. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U-test. P ≤ 0.05 was regarded as significant (*P ≤ 0.05 and ** P ≤ 0.01).
Farming environment is related to lower proportions of FOXP3+CD25high T cells in early infancy
As we found a significant difference regarding the incidence of allergic disease between children who lived on dairy farms and children who lived on the countryside but not on dairy farms, we next examined whether there were differences between these two groups of children regarding the various T cell variables during the first 3 years of life. The OPLS-DA score scatter plot in Fig.5a displays a clear distinction between farmers' children (filled circled) and non-farmers' children (open circles), with respect to the T cell variables described above.
Figure 5.
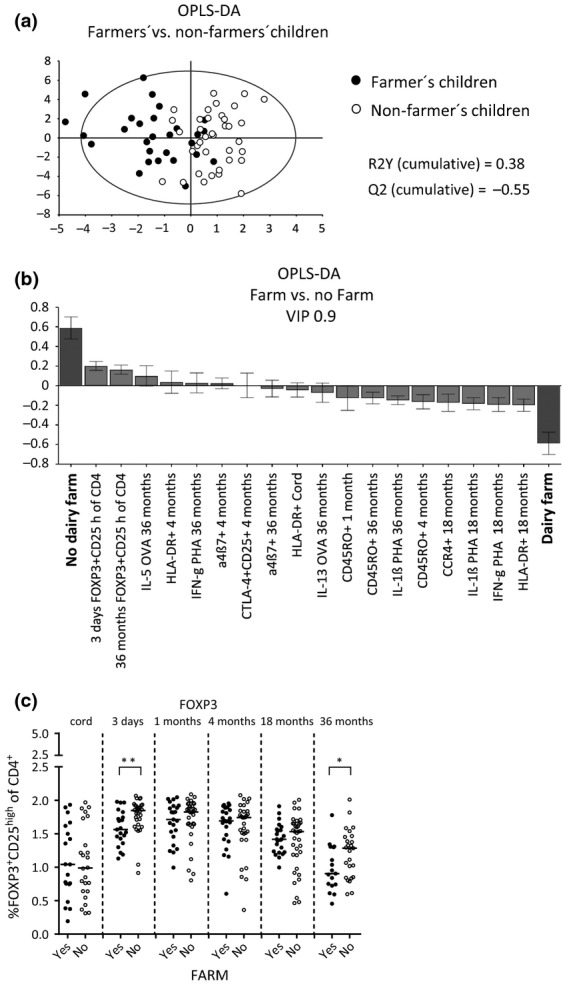
(a) OPLS discriminant analysis (OPLS-DA) score scatter plot displaying the separation between farmers' (filled circles) and non-farmers' children (open circles) and X-variables, including CD4+ T cells that were FOXP3+CD25high, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ at birth, 3–5 days, and 1, 4, 18 and 36 months of age. Measurements of PHA-induced cytokine production by mononuclear cells at 4, 18 and 36 months and OVA- and birch allergen extract-induced cytokine production at 36 months of age were also included in the analyses. (b) OPLS-DA loadings column plots that depicts the associations between the X-variables and Y, a farm or a non-farm environment. X-variables with bars projected in the same direction as Y are positively associated, whereas parameters in the opposite direction are inversely related to Y. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The OPLS-DA loadings column plot is based on X-variables with VIP values ≥ 0.9. R2Y indicates how well the variation of Y is explained, while Q2 indicates how well Y can be predicted. R2Y = 0.28, Q2 = 0.16. (c) The proportions of FOXP3+CD25high cells within the CD4+ T cell population during the first 3 years of life in farmers' and non-farmers' children. Each dot represents an individual, and horizontal bars indicate median values. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U-test. P ≤ 0.05 was regarded as significant (*P ≤ 0.05 and ** P ≤ 0.01).
As seen in Fig.5b, high proportions of FOXP3+CD25high T cells at 3 days as well as at 36 months of age were positively associated with growing up in a non-farming environment. Univariate analyses showed that children from farming and non-farming families had comparable proportions of FOXP3+CD25high cells within the CD4+ T cell population in cord blood (Fig.5c). However, at 3 days as well as at 36 months of age, non-farmers' children had significantly higher proportions of these cells in their circulation compared with children from farming families (Fig.5c). Moreover, multivariate analysis showed that a farming environment was associated with higher proportions of CD4+ T cells that express HLA-DR, CCR4 and CD45RO as well as with a higher PHA-induced production of IFN-γ and IL-1β by mononuclear cells (Fig.5b). The OPLS-DA loadings column plot in Fig.5b is based on parameters with VIP values ≥ 0.9. A similar association pattern was observed when all allergic children were excluded from the analysis, which indicates that allergy per se is not a confounding factor for these results (data not shown). When corresponding analyses including all children were performed for FOXP3+CD25+ cells within the CD4+ T cell population, similar multivariate association patterns were found as shown in Fig.5 (data not shown). However, non-farmers' children had significantly higher proportions of FOXP3+CD25+ cells than farmers' children at 1 month of age (P = 0.03), but not at any other time-point. Taken together, these results suggest that a farming environment is related to lower proportions of FOXP3+CD25high putative Tregs within the CD4+ T cell population in early infancy and also later in childhood.
Discussion
The relationship between the proportions of regulatory T cells early in life and development of sensitization and/or allergic disease later in childhood has not yet been resolved. The present birth cohort study demonstrates that children who were sensitized at 18 or 36 months of age had a higher proportion of FOXP3+ CD25high T cells within the CD4+ T cell population at birth and at 3 days of life than children who remained non-sensitized. We also show that exposure to a dairy farm environment was associated with lower proportions of FOXP3+CD25high T cells in early infancy. Thus, our results demonstrate that a high proportion of FOXP3+CD25high T cells within the CD4+ T cell population at birth and in early infancy do not prevent the atopic march. On the contrary, a high proportion of FOXP3+CD25high T cells this early in life may prevent activation and necessary maturation of the immune system, thereby favouring faulty programming that may lead to sensitization in children.
FOXP3+ Tregs are important for the maintenance of immunological tolerance as children with mutations in FOXP3 develop autoimmunity, severe food allergy and hyper-IgE levels in blood 4,28. Based on these findings, it has been suggested that FOXP3+ Tregs are instrumental in the prevention of sensitization and allergic diseases 29. Interestingly, we here demonstrate for the first time that children who were sensitized at 18 or 36 months of age had higher proportions of FOXP3+CD25high T cells within the CD4+ T cell population at birth and early infancy than non-sensitized children. These associations were also true for the proportions of neonatal FOXP3+CD25+ T cells. These results are in discordance with a recent study in which the fraction of FOXP3+CD25+ T cells at birth was unrelated to sensitization at both 1 and 2 years of age 16. One explanation for this discrepancy may be that we have examined the proportions of FOXP3+CD25high and FOXP3+CD25+ cells within the CD4+ T cell population, while McLoughlin et al. studied the fraction of CD4+CD25+FOXP3+ within the CD3+ T cell population, which includes both CD4+ and CD8+ T cells. Another putative source of difference is that McLoughlin et al. examined inner city children with a parental history of allergic disease or asthma, whereas we investigated children from rural areas of whom only one-third had allergic parents.
Contrary to sensitization, a clinical diagnosis of allergy did not show any significant association with high proportions of either FOXP3+CD25high or FOXP3+CD25+ T cells at birth. In accordance with this, others have shown that the fraction of FOXP3+CD25+ cells within the CD3+ or CD4+ T cell population, respectively, at birth did not differ between children who developed eczema at either 1 or 2 years of age and children who did not 16,17. Further, the proportion of Tregs defined as CD25+CD127low/neg cells at birth was unrelated to doctors' diagnosed egg allergy at 1 year of age 15. It should be noted that allergic sensitization at 18 months has been shown to be a predictor for wheeze, asthma and rhinitis, but not eczema, at 5 years of age 3. Eczema was the dominating symptom of allergic children in the present cohort, as often the case in young children. Thus, a relation between high proportions of neonatal FOXP3+CD25high T cells and allergic disease in children older than 3 years, when the prevalence of eczema is lower 30, should not be ruled out.
Multivariate analysis also demonstrated that a clinical diagnosis of allergy at 36 months was associated with higher proportions of FOXP3+CD25+ cells but not with FOXP3+CD25high cells within the CD4+ T cell population at both 18 and 36 months of age. These findings are in agreement with a previously observed expansion of the circulating fraction of CD4+CD25+FOXP3+ cells within the CD3+ T cell population in children presenting with eczema at 2 years of age compared with non-allergic controls 16. Thus, higher proportions of FOXP3+CD25+, but not FOXP3+CD25high, T cells within the CD4+ T cell population just before or during established allergic disease suggest an expansion of activated cells within the FOXP3+CD25+ T cell subset in response to the allergic inflammation.
In the present study, children in the farming group had a lower incidence of allergy, while there was no significant effect of farming on sensitization. This is in accordance with previous studies showing that farming is protective against sensitization but has a more dramatic protective effect against clinical allergy 19. Given the small size of our study, it is not surprising that a modest protective effect of farming on sensitization was not evident. Nevertheless, although exposure to a farming environment had an allergy-protective effect, this was not a confounding factor for the associations between higher proportions of FOXP3+CD25high T cells at birth and early in infancy and subsequent sensitization in the present study.
To our knowledge, it is not known whether the post-natal T cell activation progress differs between farmers' and non-farmers' children. Here, we demonstrate that farmers' children had significantly lower proportions of FOXP3+CD25high cells early in infancy as well as later in childhood compared with non-farmers' children, while the proportions of FOXP3+CD25+ cells within the CD4+ T cell population solely differed at 1 month of age. In contrast, it was recently shown that farmers' children had significantly higher proportions of CD4+CD25highCD127low/− cells within the total lymphocyte population at 4.5 years of age than non-farmers' children 31. However, they also found that the proportions of CD4+CD25+FOXP3+ cells within the total lymphocyte population did not differ between these two groups of children at this age. Interestingly, upon stimulation with PMA/ionomycin or LPS in vitro, they found that the proportions of CD4+CD25+FOXP3+ cells, but not CD4+CD25highCD127low/− cells, within the total lymphocyte population increased significantly among farmers' compared with non-farmers' children 31. In conclusion, their results indicate that the CD25+ T cell subset may also include activated cells. This is in line with our speculations that the FOXP3+CD25+ cell subset in our study may include activated non-regulatory T cells.
Here, we also found that a farming environment was related to a more pronounced T cell memory conversion and cytokine production. Therefore, we hypothesize that a low proportion of neonatal FOXP3+CD25high cells within the CD4+ T cell population in farmers' children may permit a more efficient post-natal T cell activation process, which could be an early sign of healthy immune maturation. However, a high proportion of FOXP3+CD25high T cells and low T cell reactivity among children in a non-faming milieu could, as discussed above, be a sign of reaction to faulty immune regulation and immune immaturity.
Of interest, newborn children from Austria and Australia, representing environments with low microbial exposure, have significantly higher proportions of putative Tregs defined as CD4+CD25high and CD25+FOXP3+CD127low, respectively, than newborns from Gabon and Papua New Guinea, representing high microbial exposure environments 32,33. As the differences were found already at birth, these results suggest that prenatal exposure to environmental antigens may influence the proportions of Tregs. However, a farming environment did not seem to affect the fetal fraction of FOXP3+CD25high T cells, as no difference regarding the proportions of these cells at birth was found between farmers' and non-farmers' children in our study. To affect prenatal proportions of FOXP3+CD25high T cells, environmental microbial load might have to reach a considerable magnitude, which may not occur at a Swedish dairy farm.
One limitation of the present study is the relatively low number of study subjects. However, the small size of this study permitted a detailed prospective follow-up with immunological analyses and careful diagnosis of sensitization and allergy by study physicians, and the study was still large enough to generate statistically significant results. Another limitation is that CD127 was not included in the flow cytometry panel as a complement to identify putative Tregs, as this marker was not discovered when the present study was initiated 34. Further, the regulatory capacity of the FOXP3+ putative Tregs could not be examined as staining for this marker requires permeabilization, and isolated Tregs would thus not be viable. All the flow cytometry analyses were performed before we obtained the data regarding clinical status of the children or whether they lived on a dairy farm or not. A further strength of this study is the use of multivariate factor analysis to find patterns and trends in the large data set, before proceeding with univariate analyses of the X-variables that contributed most to the respective models.
Further studies are required to confirm that high proportions of putative Tregs at birth and early in infancy precede sensitization and that high proportions of these cells early in life may impede the post-natal T cell maturation process. The potential difference in the regulatory capacity/activity of these cells during the first months of life also needs to be examined. It also remains to be elucidated whether higher proportions of FOXP3+CD25high cells within the CD4+ T cell population in cord blood are a consequence of the environment in utero, genetic factors or a combination of both.
Acknowledgments
We thank study nurses Anders Nordberg and Helen Andersson at Skaraborgs Hospital, Skövde and Lidköping, respectively, as well as the paediatricians Margareta Ceder, Gunhild Lindhagen and Carl-Johan Törnhage at the Pediatric Clinics at Vara Medical Center, Falköping Hospital, and Skaraborg Hospital in Skövde, Sweden, respectively. We highly appreciate the skilful technical assistance of Inger Nordström, along with the staff at the Clinical Immunology Laboratory of the Sahlgrenska University Hospital. Finally, we thank all the families who took part in the study. This work was funded by the Swedish Research Council (Grant K2012-57X-22047-01-6), the Region Västra Götaland (agreement concerning research and education of doctors; ALF), the Torsten and Ragnar Söderberg's Foundation, The Health and Medical Care Executive Board of the region Västra Götaland, the Swedish Society of Medicine, the Göteborg Medical Society/The Swedish Order of Freemasons in Gothenburg and by the Magnus Bergvall foundation.
Conflict of interest
The authors declare no conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Variable influence on projection (VIP) plots. The VIP plots display the VIP values as a column plot sorted in descending order with confidence intervals derived from jack knifing. VIP summarizing the importance of the X-variables that contribute most (positively or negatively) to sensitization, i.e. specific IgE to food and/or inhalant allergens, at (a) 18 and (b) 36 months of age.
Figure S2. (A and B) OPLS-DA loadings column plot depicting the associations between sensitization, that is, specific IgE to food and/or inhalant allergens, at (A) 18 and (B) 36 months of age and X-variables, including CD4+ T cells that are FOXP3+CD25+, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ at birth, 3–5 days, and 1, 4, 18 and 36 months of age. Measurements of PHA-induced cytokine production by mononuclear cells at 4, 18 and 36 months and OVA- and birch allergen-extract induced cytokine production at 36 months of age were also included in the analyses. For allergy at 18 months, immune parameters measured at 36 months were not included. X-variables with bars projected in the same direction as allergy are positively associated, whereas parameters in the opposite direction are inversely related to allergy at this age. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The OPLS-DA loadings column plots are based on X-variables with VIP values ≥ (A) 0.8, (B) 1.0 R2Y indicates how well the variation of Y is explained, while Q2 indicates how well Y can be predicted. R2Y: (A) = 0.22, (B) = 0.43. Q2: (A) = 0.17, (B) = 0.29. (C–D) The proportion of FOXP3+CD25+ cells within the CD4+ T cell population among children who are sensitized or not at (C) 18 or (D) 36 months of age. Each dot represents an individual, and horizontal bars indicate median value. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U -test. P ≤ 0.05 was regarded as significant (***P ≤ 0.001).
Table S1. Cytokine production by mononuclear cells upon in vitro stimulation with PHA, birch allergen extract or ovalbumin at 4, 18 or 36 months of age. Data represent the median value and the range is shown in brackets.
References
- Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–6. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15:627–33. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Almqvist C, Li Q, Britton WJ, et al. Early predictors for developing allergic disease and asthma: Examining separate steps in the ‘allergic march’. Clin Exp Allergy. 2007;37:1296–302. doi: 10.1111/j.1365-2222.2007.02796.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–25. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindebacke H, Stenstad H, Quiding-Järbrink M, et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol. 2009;183:4360–70. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Mjosberg J, Svensson J, Johansson E, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183:759–69. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- Hinz D, Bauer M, Roder S, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67:380–9. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/-) regulatory T cell function. J Allergy Clin Immunol. 2008;121:1460–6. doi: 10.1016/j.jaci.2008.03.025. 1466 e1-7. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Calatroni A, Visness CM, et al. Longitudinal relationship of early life immunomodulatory T cell phenotype and function to development of allergic sensitization in an urban cohort. Clin Exp Allergy. 2012;42:392–404. doi: 10.1111/j.1365-2222.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Lou H, Wang C, et al. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol. 2013;24:178–86. doi: 10.1111/pai.12050. [DOI] [PubMed] [Google Scholar]
- Matricardi PM. Infections preventing atopy: facts and new questions. Allergy. 1997;52:879–82. doi: 10.1111/j.1398-9995.1997.tb01246.x. [DOI] [PubMed] [Google Scholar]
- Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- Williams HC. Diagnostic criteria for atopic dermatitis. Lancet. 1996;348:1391–2. doi: 10.1016/S0140-6736(05)65466-9. [DOI] [PubMed] [Google Scholar]
- Rabe H, Lundell AC, Andersson K, Adlerberth I, Wold AE, Rudin A. Higher proportions of circulating FOXP3+ and CTLA-4+ regulatory T cells are associated with lower fractions of memory CD4+ T cells in infants. J Leukoc Biol. 2011;90:1133–40. doi: 10.1189/jlb.0511244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun. 2002;70:6688–96. doi: 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Böhm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–93. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Linane A, Moes N, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–17. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Curotto deLafailleMA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–8. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105:99–106. doi: 10.1016/j.anai.2009.10.002. quiz 107-9, 117. [DOI] [PubMed] [Google Scholar]
- Lluis A, Depner M, Gaugler B, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014;133:551–9. doi: 10.1016/j.jaci.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Kohler C, Adegnika AA, Van der Linden R, et al. Comparison of immunological status of African and European cord blood mononuclear cells. Pediatr Res. 2008;64:631–6. doi: 10.1203/PDR.0b013e31818718ba. [DOI] [PubMed] [Google Scholar]
- Lisciandro JG, Prescott SL, Nadal-Sims MG, et al. Comparison of neonatal T regulatory cell function in Papua New Guinean and Australian newborns. Pediatr Allergy Immunol. 2012;23:173–80. doi: 10.1111/j.1399-3038.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Variable influence on projection (VIP) plots. The VIP plots display the VIP values as a column plot sorted in descending order with confidence intervals derived from jack knifing. VIP summarizing the importance of the X-variables that contribute most (positively or negatively) to sensitization, i.e. specific IgE to food and/or inhalant allergens, at (a) 18 and (b) 36 months of age.
Figure S2. (A and B) OPLS-DA loadings column plot depicting the associations between sensitization, that is, specific IgE to food and/or inhalant allergens, at (A) 18 and (B) 36 months of age and X-variables, including CD4+ T cells that are FOXP3+CD25+, CTLA-4+CD25+, CD45RO+, HLA-DR+, CCR4+ or α4β7+ at birth, 3–5 days, and 1, 4, 18 and 36 months of age. Measurements of PHA-induced cytokine production by mononuclear cells at 4, 18 and 36 months and OVA- and birch allergen-extract induced cytokine production at 36 months of age were also included in the analyses. For allergy at 18 months, immune parameters measured at 36 months were not included. X-variables with bars projected in the same direction as allergy are positively associated, whereas parameters in the opposite direction are inversely related to allergy at this age. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. The OPLS-DA loadings column plots are based on X-variables with VIP values ≥ (A) 0.8, (B) 1.0 R2Y indicates how well the variation of Y is explained, while Q2 indicates how well Y can be predicted. R2Y: (A) = 0.22, (B) = 0.43. Q2: (A) = 0.17, (B) = 0.29. (C–D) The proportion of FOXP3+CD25+ cells within the CD4+ T cell population among children who are sensitized or not at (C) 18 or (D) 36 months of age. Each dot represents an individual, and horizontal bars indicate median value. Statistical differences between the groups were calculated using two-tailed Mann–Whitney U -test. P ≤ 0.05 was regarded as significant (***P ≤ 0.001).
Table S1. Cytokine production by mononuclear cells upon in vitro stimulation with PHA, birch allergen extract or ovalbumin at 4, 18 or 36 months of age. Data represent the median value and the range is shown in brackets.


