Abstract
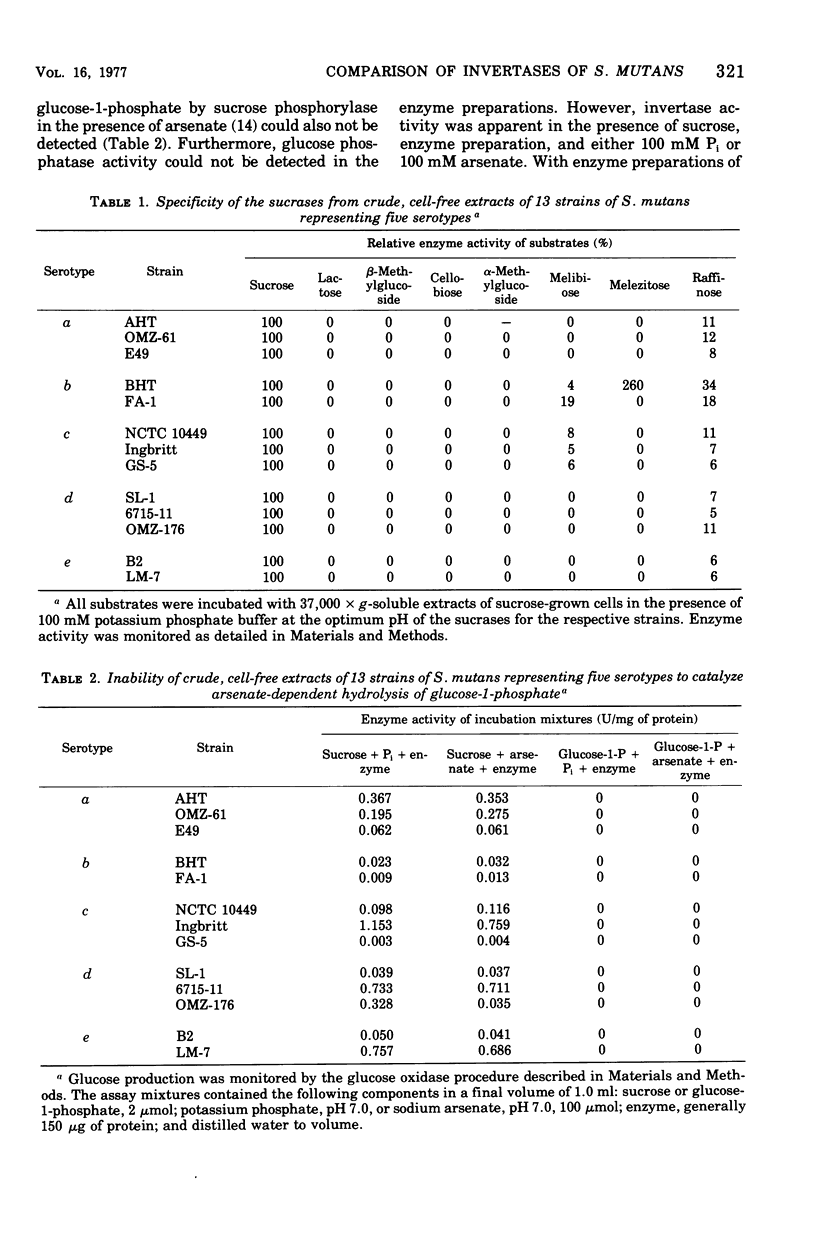
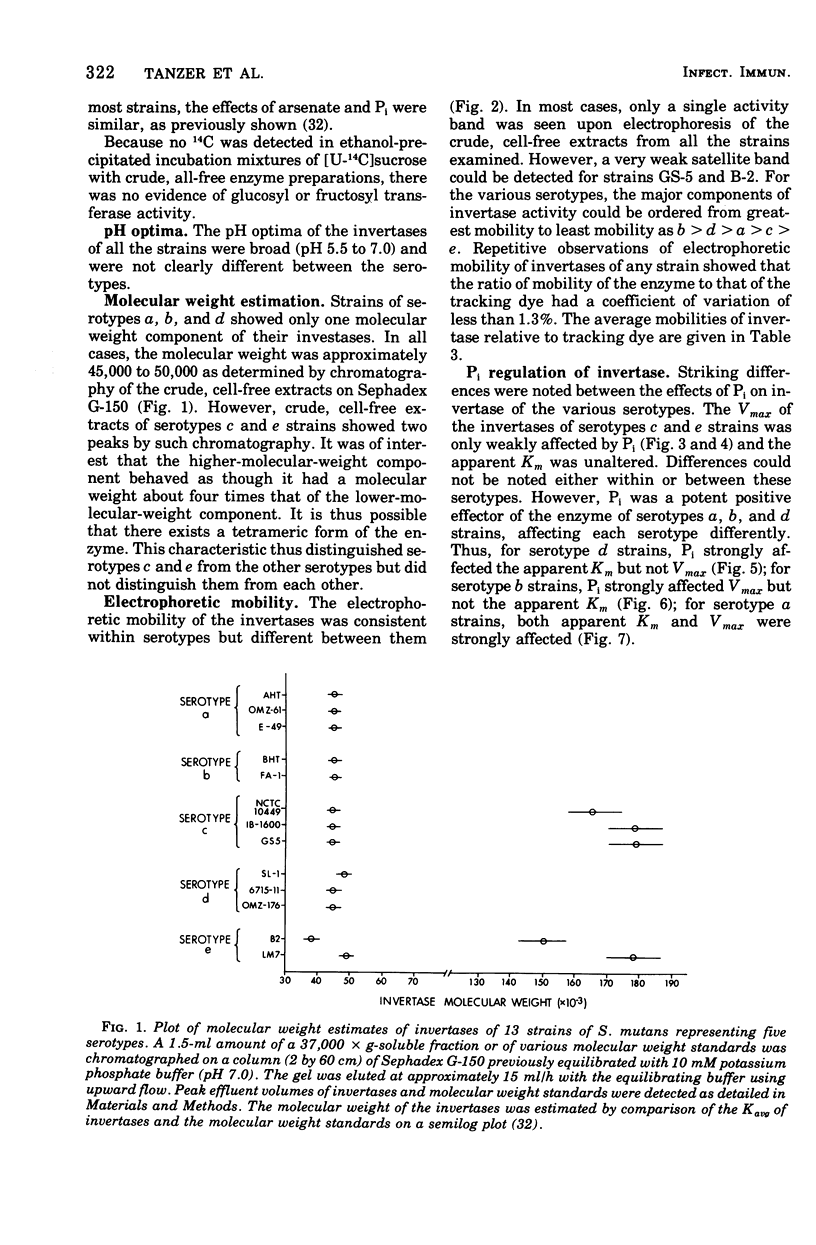
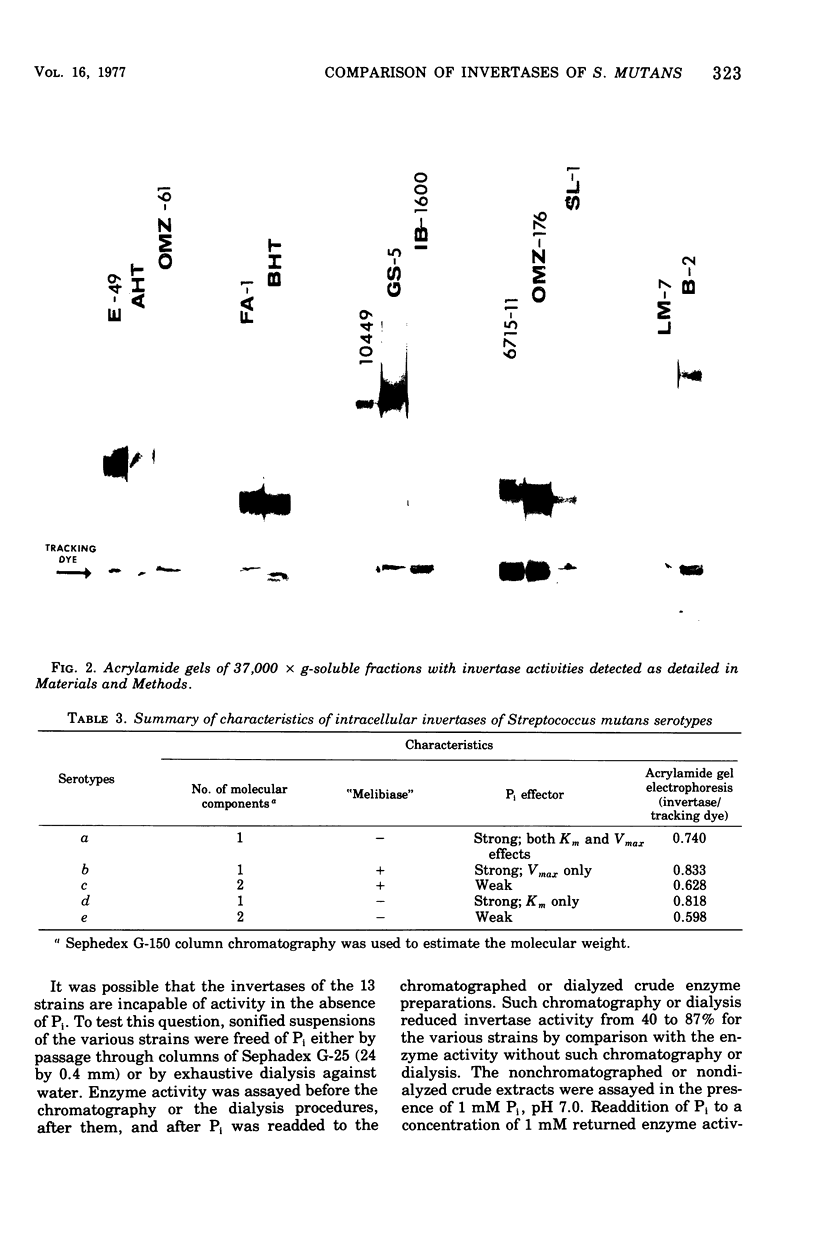
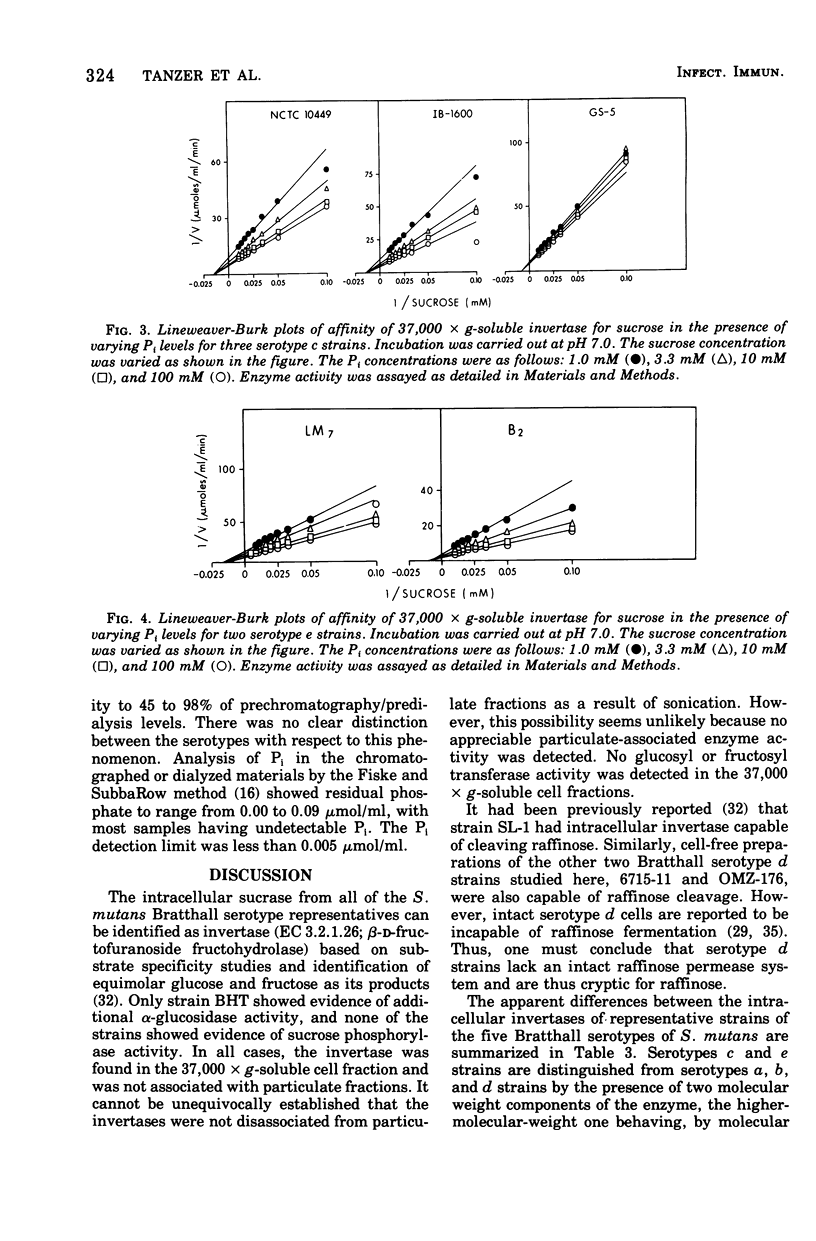
Sucrase activity was studied in 13 strains of Streptococcus mutans representing the five Bratthall serotypes. Sucrose-adapted cells have sucrase activity in the 37,000 × g-soluble fraction of all strains. The enzyme was identified as invertase (β-d-fructofuranoside fructohydrolase; EC 3.2.1.26) because it hydrolyzed the β-fructofuranoside trisaccharide raffinose, giving fructose and melibiose as its products, and because it hydrolyzed the β-fructofuranoside dissacharide sucrose, giving equimolar glucose and fructose as its products. Invertases of c and e strains exhibit two activity peaks by molecular exclusion chromatography with molecular weights of 45,000 to 50,000 and about 180,000; those of serotypes a, b, and d strains exhibit only a single component of 45,000 to 50,000 molecular weight. The electrophoretic mobility of invertases is different between the serotypes and the same within them. Inorganic orthophosphate (Pi) has a weak positive effect on the Vmax of invertases of serotypes c and e cells but a strong positive effect on the invertases of serotype b cells; Pi has a strong positive effect on the apparent Km of the invertases of serotype d cells, but has no effect on the Vmax; Pi has a strong positive effect on both the apparent Km and Vmax of the invertases of serotype a cells. Thus, the invertases were different between all of the serotypes but similar within the serotypes. These findings support the taxonomic schemes of Coykendall and of Bratthall. It was additionally noted that 37,000 × g-soluble fractions of only serotypes b and c but not serotypes a, d, and e cells have melibiase activity, and it could be deduced that serotype d cells lack an intact raffinose permease system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Heterogeneity of Streptococcus mutans strains based on their mannitol-1-phosphate dehydrogenases: criterion for rapid classification. Infect Immun. 1972 Sep;6(3):422–424. doi: 10.1128/iai.6.3.422-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham J. C., Hageage G. J., Jr Adenosine phosphate hydrolases in cell fractions of Vitreoscilla. J Bacteriol. 1967 Jan;93(1):191–198. doi: 10.1128/jb.93.1.191-198.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Hageage G. J., Jr, Wittenberger C. L. Multicomponent nature of the glucosyltransferase system of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C87–C96. doi: 10.1177/002203457605500330011. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Base composition of deoxyribonucleic acid isolated from cariogenic streptococci. Arch Oral Biol. 1970 Apr;15(4):365–368. doi: 10.1016/0003-9969(70)90063-4. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Bratthall D., O'Connor K., Dvarskas R. A. Serological and genetic examination of some nontypical Streptococcus mutans strains. Infect Immun. 1976 Sep;14(3):667–670. doi: 10.1128/iai.14.3.667-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Hausner T., Clewell D. B. Buoyant densities of DNA from various strains of Streptococcus mutans. Arch Oral Biol. 1972 Jun;17(6):1001–1003. doi: 10.1016/0003-9969(72)90123-9. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Cell wall and serological studies on Streptococcus mutans. Caries Res. 1974;8(4):301–316. doi: 10.1159/000260120. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J. A biochemical scheme for the separation of the five varieties of Streptococcus mutans. Arch Oral Biol. 1974 Nov;19(11):1079–1081. doi: 10.1016/0003-9969(74)90099-5. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M. Studies on the fate of the glucosyl moiety of sucrose metabolized by Streptococcus mutans. J Dent Res. 1972 Mar-Apr;51(2):415–423. doi: 10.1177/00220345720510023001. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Wood W. I., Krichevsky M. I. Linear growth kinetics of plaque-forming streptococci in the presence of sucrose. J Gen Microbiol. 1969 Sep;58(1):125–133. doi: 10.1099/00221287-58-1-125. [DOI] [PubMed] [Google Scholar]
- Thomson L. A., Little W., Hageage G. J. Application of fluorescent antibody methods in the analysis of plaque samples. J Dent Res. 1976 Jan;55:A80–A86. doi: 10.1177/002203457605500126011. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Direct microdetermination of sucrose. Anal Biochem. 1968 Feb;22(2):280–283. doi: 10.1016/0003-2697(68)90317-5. [DOI] [PubMed] [Google Scholar]



