Abstract
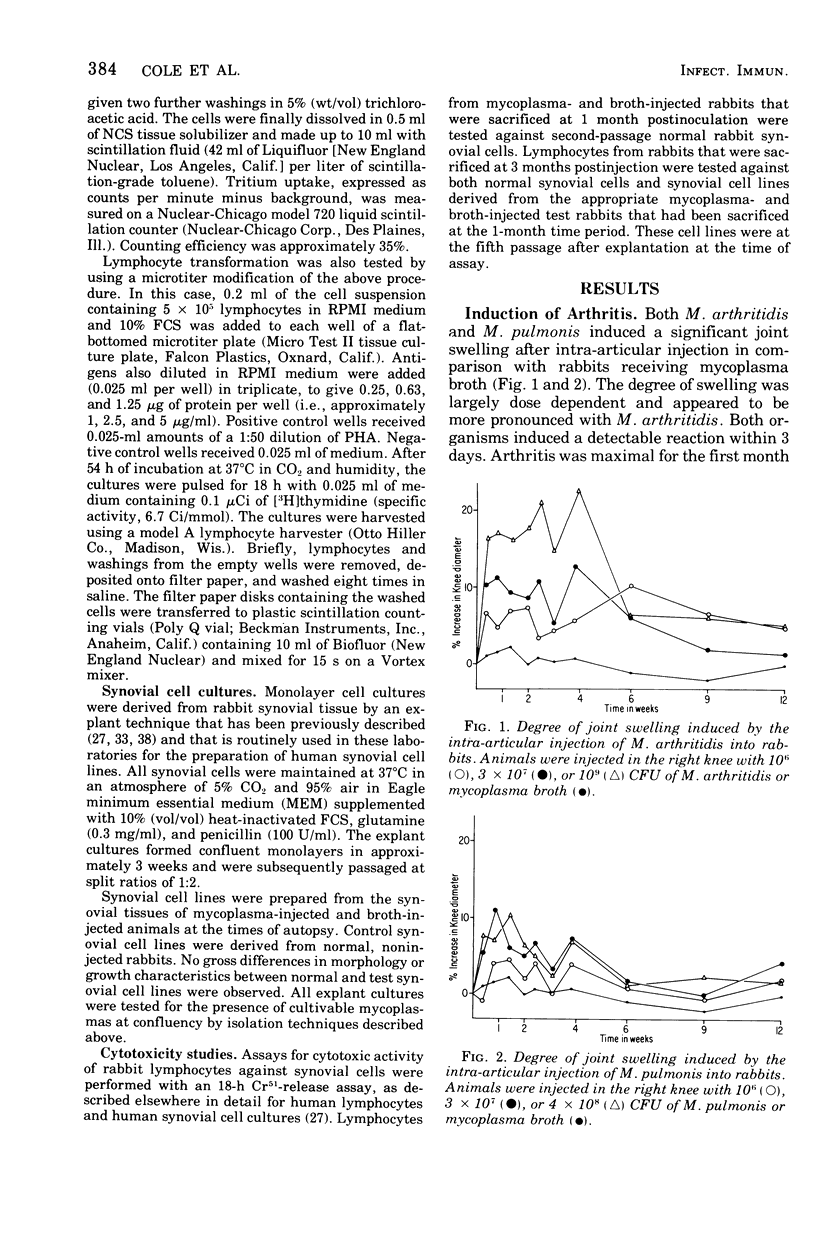
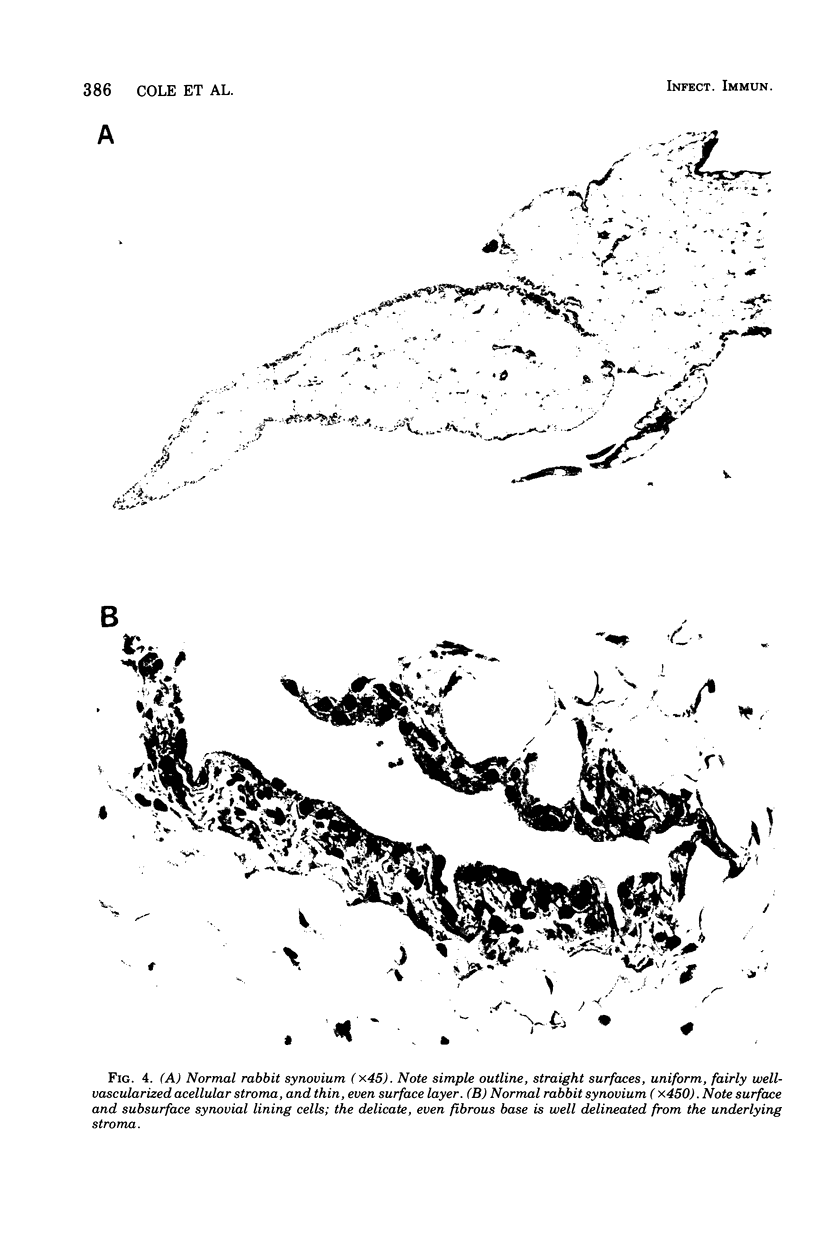
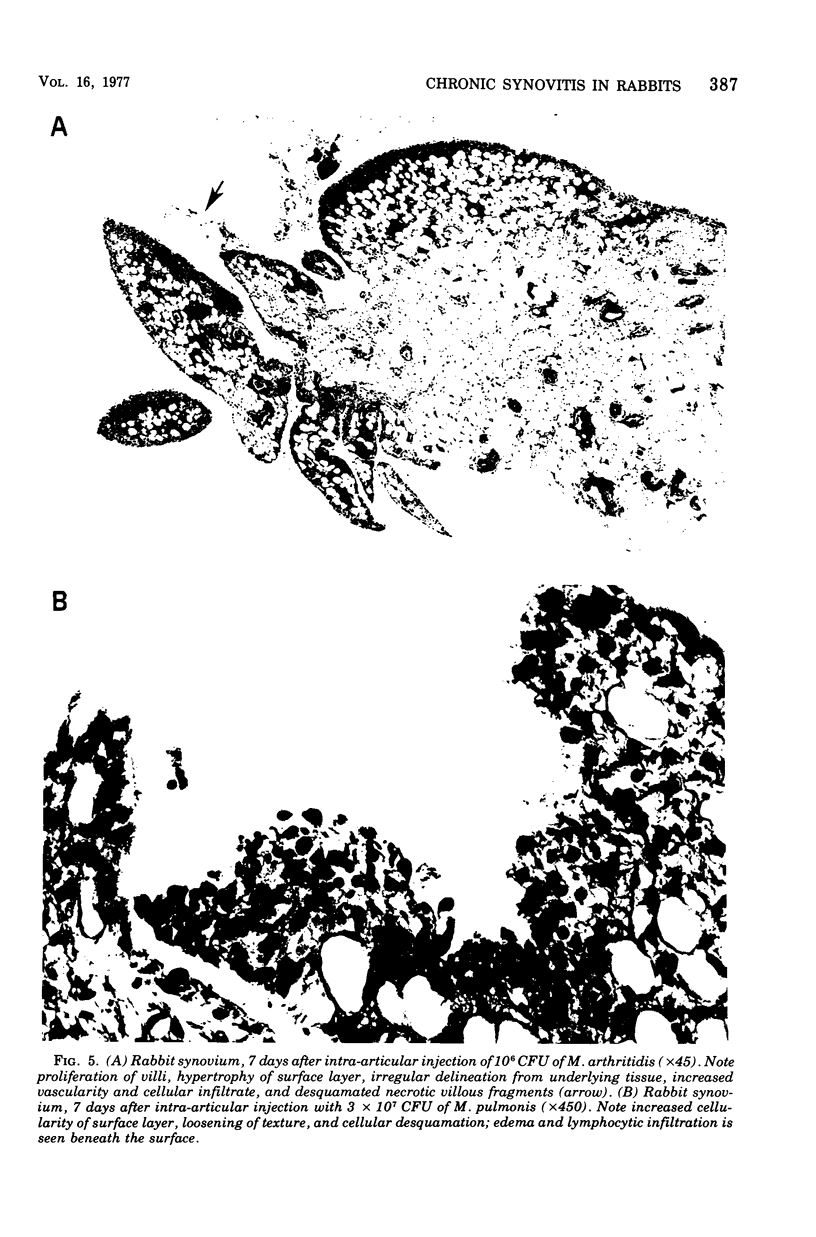
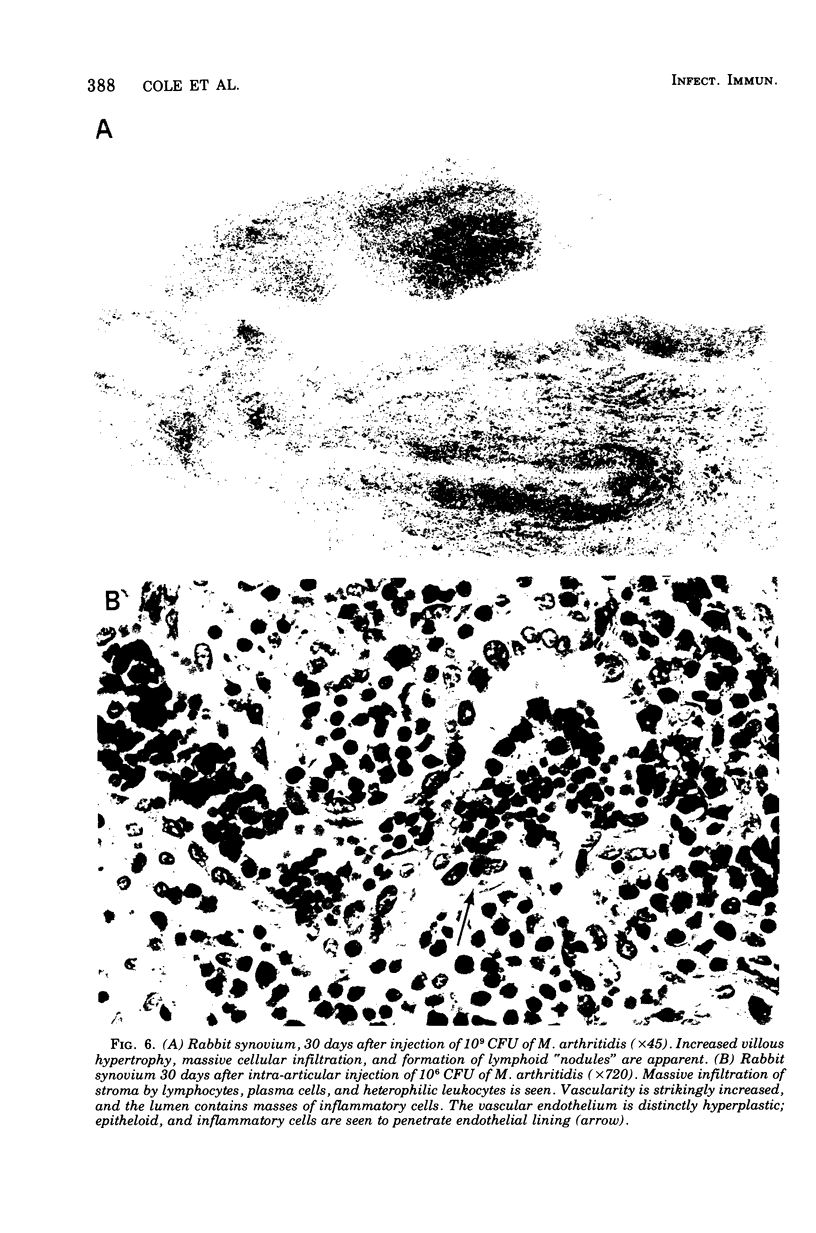
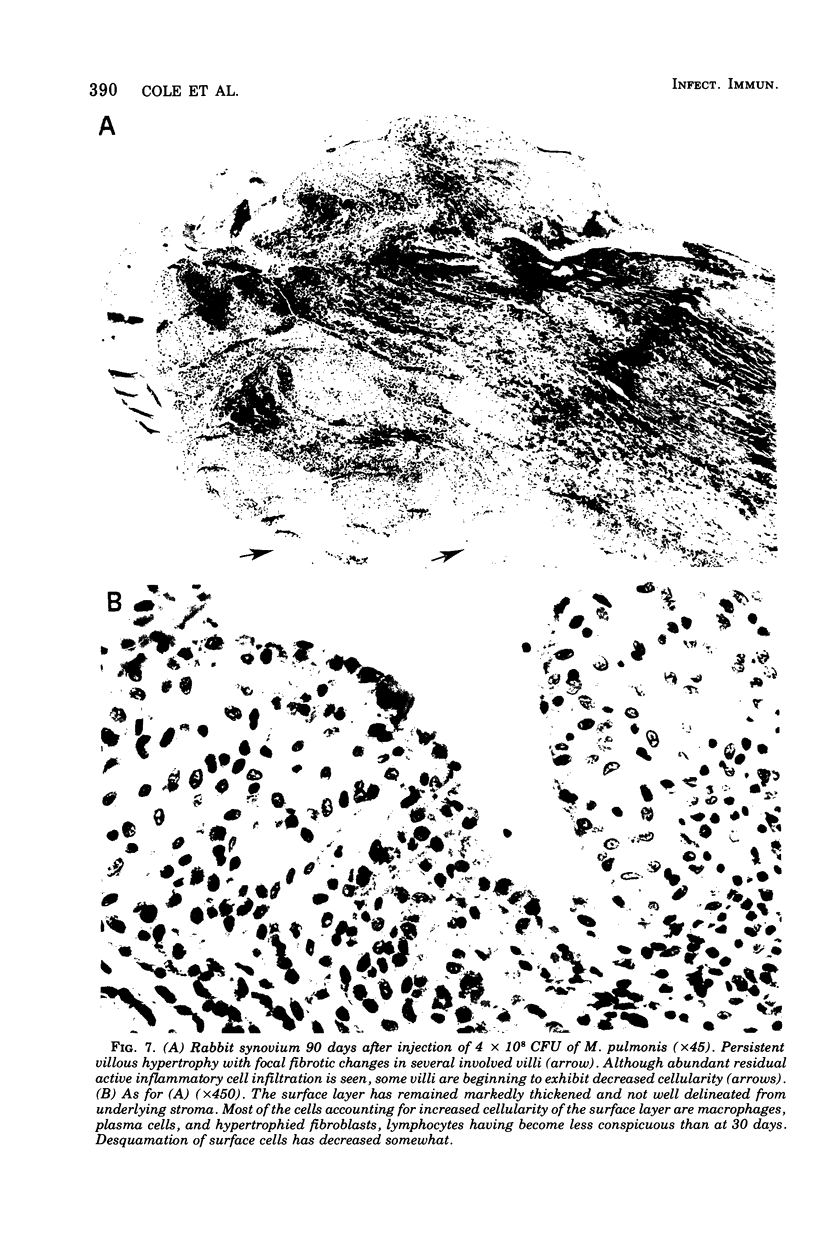
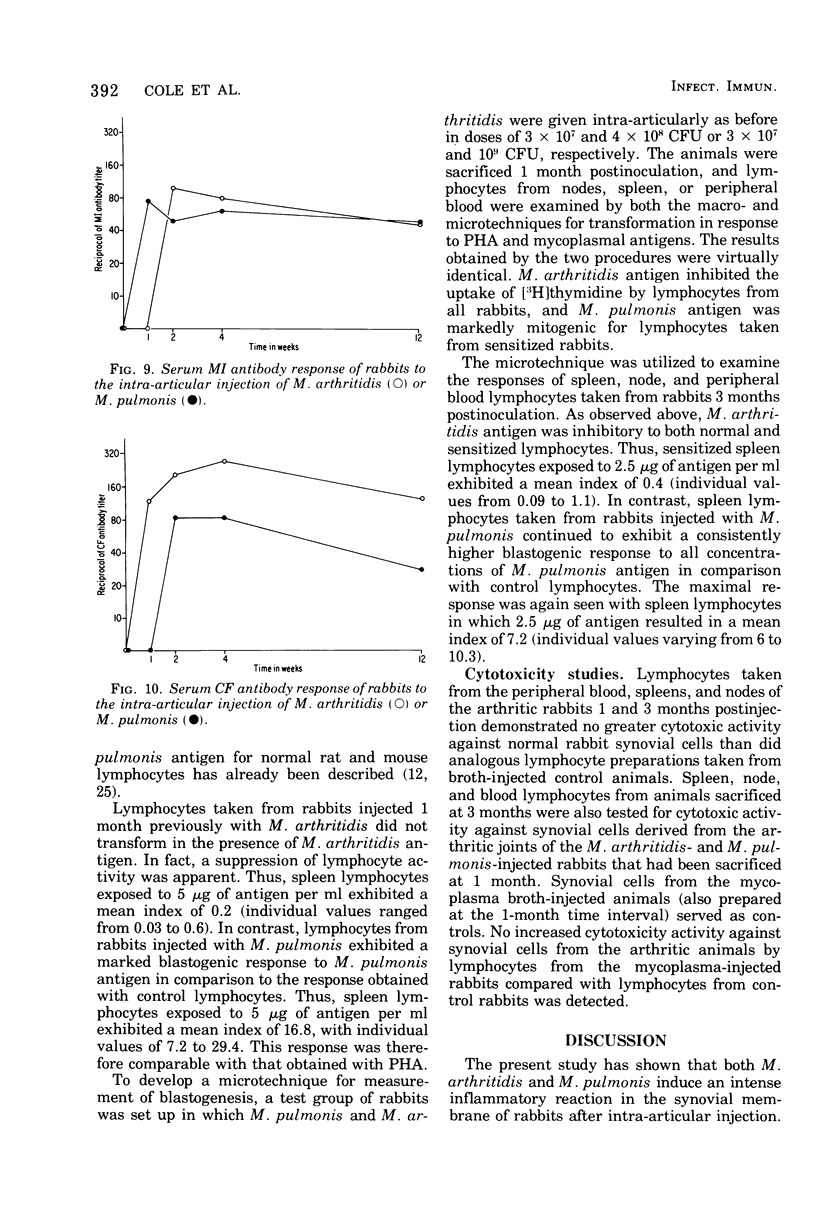
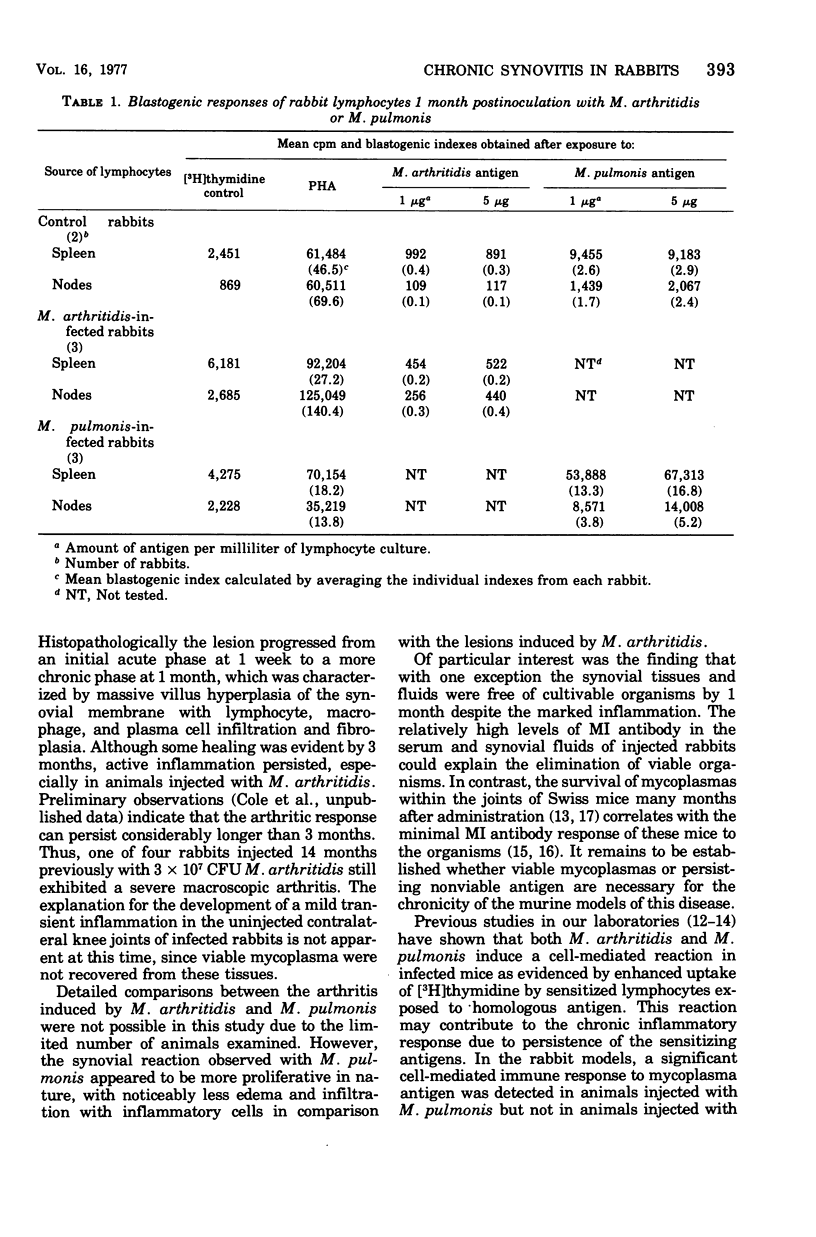
A dose-dependent chronic synovitis was induced in rabbit knees after the intra-articular injection of both Mycoplasma arthritidis and Mycoplasma pulmonis. The inflammation progressed from an initial acute phase at 1 week characterized by edema, infiltration of the synovium with monocytes and heterophils, and desquamation of lining cells, to a more chronic phase at 1 and 3 months, in which villus hyperplasia, lymph "nodules," mononuclear cell infiltration, fibroplasia, and collagen deposition were prominent. With one exception, mycoplasmas could no longer be cultivated from the joints 1 month postinoculation. Both mycoplasma species evoked a humoral antibody response that was more marked in synovial fluids than in peripheral blood. A cell-mediated immune reaction, as evidence by enhanced uptake by [3H]thymidine by sensitized blood, spleen, or node lymphocytes in the presence of homologous antigen, was detected only in rabbits injected with M. pulmonis. Lymphocytes taken from arthritic rabbits were no more cytotoxic toward synovial cells derived from normal or arthritic rabbits than were normal lymphocytes. The models of synovitis described in this study offer a convenient probe for determining the mechanisms of mycoplasma-induced inflammation, since they require only a single injection of the initiating agent and, in addition, utilize an animal host large enough for detailed investigation into the nature of mycoplasma/synovium interactions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreis M., Stastny P., Ziff M. Experimental arthritis produced by injection of mediators of delayed hypersensitivity. Arthritis Rheum. 1974 Sep-Oct;17(5):537–551. doi: 10.1002/art.1780170508. [DOI] [PubMed] [Google Scholar]
- Astorga G. P. Immunological studies of an experimental chronic arthritis resembling rheumatoid arthritis. Arthritis Rheum. 1969 Dec;12(6):589–596. doi: 10.1002/art.1780120606. [DOI] [PubMed] [Google Scholar]
- Bacon P. A., Bluestone R., Goldberg L. S., Webb F. W., Pearson C. M., Cooke M., Stevens J. G. Experimental herpes virus arthritis. Factors in chronicity. Ann Rheum Dis. 1974 Sep;33(5):413–421. doi: 10.1136/ard.33.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden J. A., Decker J. L. Mycoplasma hyorhinis swine arthritis. I. Clinical and microbiologic features. Arthritis Rheum. 1971 Mar-Apr;14(2):193–201. doi: 10.1002/art.1780140202. [DOI] [PubMed] [Google Scholar]
- Barden J. A., Tully J. G. Experimental arthritis in mice with Mycoplasma pulmonis. J Bacteriol. 1969 Oct;100(1):5–10. doi: 10.1128/jb.100.1.5-10.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist L. M., Lau B. H., Winter C. E. Mycoplasma-associated immunosuppression: effect on hemagglutinin response to common antigens in rabbits. Infect Immun. 1974 Feb;9(2):410–415. doi: 10.1128/iai.9.2.410-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Cole B. C., Wiley B. B., Ward J. R. Role of Biological Mimicry in the Pathogenesis of Rat Arthritis Induced by Mycoplasma arthritidis. Infect Immun. 1971 Jan;3(1):24–35. doi: 10.1128/iai.3.1.24-35.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coke B. C., Ward J. R., Golightly-Rowland C., Trapp G. A. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. II. Serological responses of the lost and effect of vaccines. Infect Immun. 1971 Oct;4(4):431–440. doi: 10.1128/iai.4.4.431-440.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R. Arthritis of mice induced by Mycoplasma pulmonis: humoral antibody and lymphocyte responses of CBA mice. Infect Immun. 1975 Nov;12(5):1083–1092. doi: 10.1128/iai.12.5.1083-1092.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis: demonstration of a cell-mediated immune response to mycoplasma antigens in vitro. Infect Immun. 1975 May;11(5):1159–1161. doi: 10.1128/iai.11.5.1159-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R., Wiley B. B. Immunological response of rodents to murine mycoplasmas. Infect Immun. 1970 Oct;2(4):419–425. doi: 10.1128/iai.2.4.419-425.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., DeWitt C. W. Mixed lymphocyte blastogenesis in response to multiple histocompatibility antigens. J Immunol. 1969 Jan;102(1):107–116. [PubMed] [Google Scholar]
- Cooke T. D., Jasin H. E. The pathogenesis of chronic inflammation in experimental antigen-induced arthritis. I. The role of antigen on the local immune response. Arthritis Rheum. 1972 Jul-Aug;15(4):327–337. doi: 10.1002/art.1780150402. [DOI] [PubMed] [Google Scholar]
- Copperman R., Morton H. E. Reversible inhibition of mitosis in lymphocyte cultures by non-viable Mycoplasma. Proc Soc Exp Biol Med. 1966 Dec;123(3):790–795. doi: 10.3181/00379727-123-31605. [DOI] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Deeb B. J., Kenny G. E. Characterization of Mycoplasma pulmonis variants isolated from rabbits. I. Identification and properties of isolates. J Bacteriol. 1967 Apr;93(4):1416–1424. doi: 10.1128/jb.93.4.1416-1424.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Nicolet J. Extensive transformation of lymphocytes by a mycoplasma organism. Nat New Biol. 1973 Dec 5;246(153):143–146. doi: 10.1038/newbio246143a0. [DOI] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. M., Smith C. B., Ward J. R., Klauber M. R. Cytotoxic activity of rheumatoid and normal lymphocytes against allogeneic and autologous synovial cells in vitro. J Clin Invest. 1976 Sep;58(3):613–622. doi: 10.1172/JCI108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Fox M. A., Guze L. B. Arthritis in mice due to infection with Mycoplasma pulmonis. I. Clinical and microbiologic features. J Infect Dis. 1973 Oct;128(4):533–540. doi: 10.1093/infdis/128.4.533. [DOI] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Fox M. A., Guze L. B. Mycoplasmal arthritis of the mouse: development of cellular hypersensitivity to normal synovial tissue. Proc Soc Exp Biol Med. 1973 Nov;144(2):561–563. doi: 10.3181/00379727-144-37635. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kaklamanis E., Pavlatos M. The immunosuppressive effect of mycoplasma infection. I. Effect on the humoral and cellular response. Immunology. 1972 Apr;22(4):695–702. [PMC free article] [PubMed] [Google Scholar]
- Krey P. R., Cohen A. S. Fine structural analysis of rabbit synovial cells. I. The normal synovium and changes in organ culture. Arthritis Rheum. 1973 May-Jun;16(3):324–340. doi: 10.1002/art.1780160306. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mansfield J. M., Wallace J. H. Incorporation of 3 H-thymidine into PHA-stimulated rabbit peripheral blood lymphocytes. Kinetics of the response. Proc Soc Exp Biol Med. 1973 Jun;143(2):408–413. doi: 10.3181/00379727-143-37332. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D. C., Chanock R. M. A color test for the measurement of antibody to the non-acid-forming human Mycoplasma species. Am J Epidemiol. 1966 Jul;84(1):51–66. doi: 10.1093/oxfordjournals.aje.a120627. [DOI] [PubMed] [Google Scholar]
- Spruance S. L., Smith C. B., Krall J., Ward J. R. Growth of Newcastle disease virus and rubella virus in rheumatoid and nonrehumatoid synovial cell cultures. Infect Immun. 1972 Sep;6(3):326–329. doi: 10.1128/iai.6.3.326-329.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P., Cooke T. D., Ziff M. Production of a macrophage migration inhibitory factor in rabbits with experimental arthritis. Clin Exp Immunol. 1973 May;14(1):141–147. [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Hensen S. A., Vincent M. M., Fuccillo D. A., Bellanti J. A. A 51 Cr microassay technique for cell-mediated immunity to viruses. J Immunol. 1973 Jun;110(6):1502–1510. [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk A., Glade P. R., Chessin L. N. Blast-like transformation induced in peripheral blood lymphocytes by cellular injury: a comparison of sonication and phytohemagglutinin. Blood. 1969 Feb;33(2):329–340. [PubMed] [Google Scholar]
- WEISSMANN G., BECHER B., WIEDERMANN G., BERNHEIMER A. W. STUDIES ON LYSOSOMES. VII. ACUTE AND CHRONIC ARTHRITIS PRODUCED BY INTRA-ARTICULAR INJECTIONS OF STREPTOLYSIN S IN RABBITS. Am J Pathol. 1965 Jan;46:129–147. [PMC free article] [PubMed] [Google Scholar]
- Webb F. W., Bluestone R., Goldberg L. S., Douglas S. D., Pearson C. M. Experimental viral arthritis induced with herpes simplex. Arthritis Rheum. 1973 Mar-Apr;16(2):241–250. doi: 10.1002/art.1780160217. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Pras M., Rosenberg L. Arthritis induced by filipin in rabbits. Arthritis Rheum. 1967 Aug;10(4):325–336. doi: 10.1002/art.1780100402. [DOI] [PubMed] [Google Scholar]
- White T. G., Puls J. L., Mirikitani F. K. Rabbit arthritis induced by cell-free extracts of erysipelothrix. Infect Immun. 1971 Jun;3(6):715–722. doi: 10.1128/iai.3.6.715-722.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]