Abstract
Aims: The role of hydrogen sulfide (H2S) in renal sodium and water homeostasis is unknown. We investigated whether H2S promoted Na+/K+-ATPase endocytosis via the H2S/EGFR/gab1/PI3K/Akt pathway in renal tubular epithelial cells. Results: H2S decreased Na+/K+-ATPase activity and induced its endocytosis in renal tubular epithelial cells, which was abrogated by small interfering RNA (siRNA) knockdown of epidermal growth factor receptor (EGFR) and gab1, a dominant-negative mutant of Akt and PI3K inhibitors. H2S increased EGFR, gab1, PI3K, and Akt phosphorylation in both renal tubular epithelial cells and kidneys of chronic salt-loaded rats. These increases were abrogated by siRNA knockdown of EGFR, but not of c-Src. Radiolabeled H2S exhibited transient, direct binding to EGFR and directly activated EGFR. Some disulfide bonds in EGFR intracellular kinase domain were susceptible to H2S-induced cleavage. Mutations of EGFR Cys797 (human) or Cys798 (rat) residues increased EGFR activity and prevented H2S-induced Na+/K+-ATPase endocytosis. H2S also inhibited sodium hydrogen exchanger-3 (NHE3) activity in renal tubular epithelial cells. H2S treatment increased sodium excretion in chronic and acute salt-loaded rats and decreased blood pressure in chronic salt-loaded rats. Innovation and Conclusion: H2S directly targets some disulfide bonds in EGFR, which activates the EGFR/gab1/PI3K/Akt pathway and subsequent Na+/K+-ATPase endocytosis and inhibition in renal tubular epithelial cells. EGFR Cys797/Cys798 residues are essential for an intrinsic inhibitory mechanism and for H2S actions in renal tubular epithelial cells. Other pathways, including NHE3, may be involved in mediating the renal effects of H2S. Our results reveal a new renal sodium homeostasis mechanism, which may provide for novel treatment approaches for diseases related to renal sodium homeostasis dysfunction. Antioxid. Redox Signal. 21, 2061–2082.
Introduction
Hydrogen sulfide (H2S) has been shown to be an endogenously generated gaseous transducer that plays an important role in regulating cardiovascular physiology and diseases. The first evidence for a physiological role of H2S was provided by Kimura et al., who showed that H2S induced vascular relaxation (10). This vasorelaxant effect was confirmed by subsequent studies in which H2S effects were attributed to the opening of KATP channels in vascular smooth muscle cells (38). In addition, H2S was shown to protect the myocardium against ischemia/reperfusion injury (2, 36), inhibit L-type calcium channels in cardiomyocytes (29), and promote angiogenesis (4, 24, 33). However, the role of H2S in renal function and salt-sensitive hypertension remains unknown.
Innovation.
Our study results reveal a new mechanism for sodium homeostasis. Hydrogen sulfide (H2S) targets epidermal growth factor receptor (EGFR) Cys797/Cys798 residues to activate the H2S/EGFR/gab1/PI3K/Akt pathway, which induces Na+/K+-ATPase endocytosis and inhibition in renal tubular epithelial cells. This not only provides the first evidence regarding a receptor for H2S to regulate renal function but also results in novel treatment approaches for diseases related to renal sodium homeostasis dysfunction, such as hypertension.
Water and sodium retention contributes to the pathogenesis of hypertension (28). The pathogenesis of salt-sensitive hypertension involves the regulation of the functions of certain ion pumps and transporters in renal tubules, where sodium reabsorption determines the balance of water and sodium in the body. Na+/K+-ATPase is a major pump for sodium reabsorption in the kidney (11). However, some sodium transport involves transporters, among which sodium hydrogen exchanger-3 (NHE3) plays a major role (14).
Na+/K+-ATPase, which pumps Na+ and K+ when it is located in the cell membrane, is regulated by endocytosis, as endocytosed Na+/K+-ATPase is inactive (16). Endocytosis of membrane proteins is a fundamental biological process occurring in various mammalian tissues. As examples, endocytosis of aquaporin-2 determines the rate of water reabsorption in renal-collecting tubes (23), and endocytosis of the LDL receptor scavenges LDL from interstitial body fluids (8). Na+/K+-ATPase in renal tubules plays an essential role in regulating sodium homeostasis in the body and is also important for the development of salt-sensitive hypertension. In this study, we investigated whether H2S was involved in Na+/K+-ATPase endocytosis regulation in renal tubular epithelial cells and sodium excretion in the kidneys of salt-loaded rats.
Na+/K+-ATPase endocytosis is regulated by endogenous ouabain that binds to the ouabain binding sites of Na+/K+-ATPase and triggers a cascade of signaling events, including binding and subsequent activation of c-Src, epidermal growth factor receptor (EGFR), gab1, and PI3K. Activation of this pathway ultimately results in the assembly of clathrin-coated pits and the subsequent endocytosis of Na+/K+-ATPase (17, 18). This prompted us to also investigate the signaling mechanisms underlying the renal effects of H2S, including the ouabain/Na+/K+-ATPase/c-Src/EGFR/gab1/PI3K signaling pathway.
Despite numerous recent studies that reported the various biological effects of H2S (34), the molecular mechanisms underlying these actions remain largely unknown. We recently identified VEGFR2 as a receptor for H2S for inducing angiogenesis in vascular endothelial cells and found that an intrinsic inhibitory Cys1045–Cys1024 disulfide bond acted as a molecular switch for H2S to regulate the structure and function of VEGFR2 (30). We hypothesized that any protein kinase which contained a functionally important disulfide bond could act as a potential receptor for H2S to mediate certain physiological functions; that is, a disulfide bond acts as a molecular switch for H2S regulation. Therefore, we also sought to identify a receptor for H2S in the ouabain/Na+/K+-ATPase/c-Src/EGFR/gab1/PI3K signaling pathway and to verify whether a certain disulfide bond acted as a molecular switch for H2S regulation. We also investigated the role of the transporter NHE3.
Results
H2S inhibits Na+/K+-ATPase activity in renal tubular epithelial cells
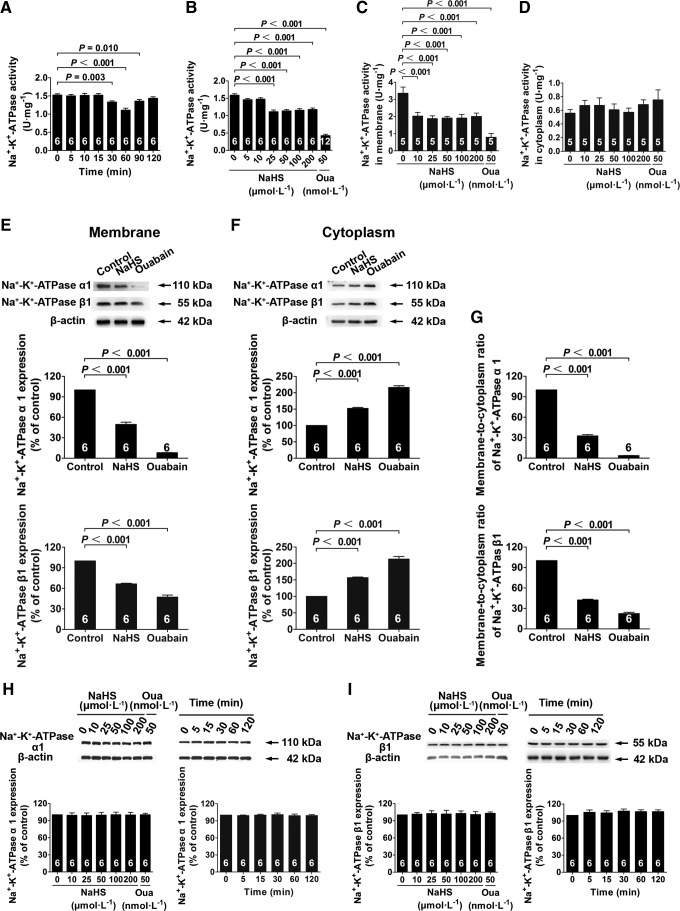
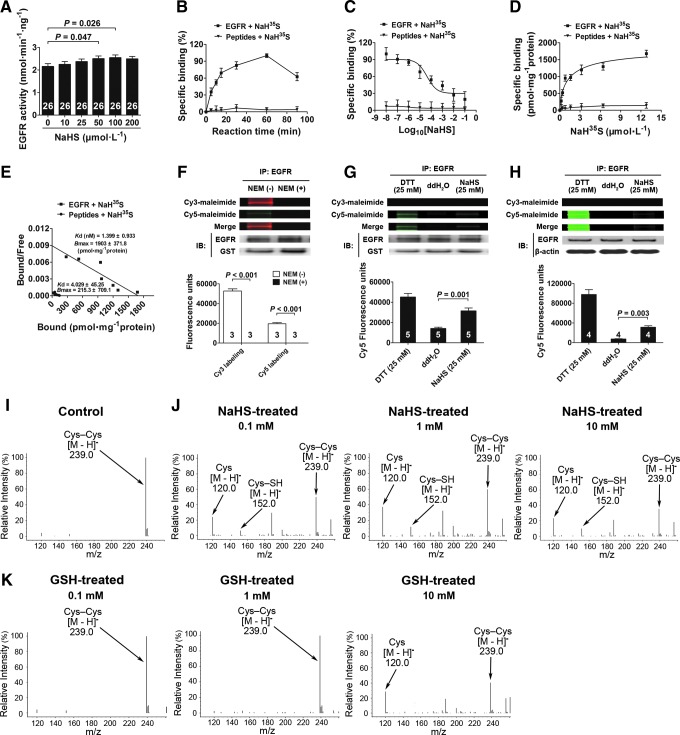
Primary renal tubular epithelial cells isolated from rats (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars) were identified by immunofluorescent staining using an antibody against cytokeratin 18 (Supplementary Fig. S1B) and by electron microscopy, in which characteristic microvilli (arrows) were observed on the surfaces of renal tubular epithelial cells (Supplementary Fig. S1C). The enzymatic activity of Na+/K+-ATPase was decreased at 30 min after adding NaHS (an H2S donor) at a concentration of 25 μM. This effect was first observed at 30 min, peaked at 60 min, and lasted until 90 min after NaHS treatment (Fig. 1A). Dose-response experiments showed that NaHS caused a decrease in Na+/K+-ATPase activity when used at concentrations of 25, 50, 100, and 200 μM at 60 min compared with vehicle treatment. These NaHS effects were comparable at concentrations ranging from 25 to 200 μM (Fig. 1B). Cells treated with ouabain (50 nM) were used as a positive control for these experiments (Fig. 1B). NaHS treatment also caused a decrease in Na+/K+-ATPase activity in the membrane fraction of renal tubular epithelial cells when used at concentrations of 10, 25, 50, 100, and 200 μM at 60 min compared with vehicle treatment (Fig. 1C). However, NaHS treatment did not change Na+/K+-ATPase activity in the cytoplasmic fraction of these cells (Fig. 1D).
FIG. 1.
H2S-induced Na+/K+-ATPase endocytosis and activity inhibition in renal tubular epithelial cells. (A, B) Cells were treated with 25 μM NaHS for different durations (A) and with different concentrations of NaHS for 60 min (B) inhibited Na+/K+-ATPase activity. (C, D) NaHS caused a decrease in Na+/K+-ATPase activity in membrane fractions at different concentrations of NaHS for 60 min (C) and had no effect in the cytoplasm fraction (D). (E–G) Isolated cell membrane (E) and cytoplasm (F) through labeling of cell surface by biotinylation and, respectively, detected the expressions of Na+/K+-ATPase α1 and β1 subunit by western blot analysis, and calculated Na+/K+-ATPase membrane-to-cytoplasm ratio (G). (H, I) H2S had no effect on Na+/K+-ATPase α1 (H) and β1 (I) subunit expression after treatment with different concentrations of NaHS for 60 min and with 25 μM NaHS for different durations. Data in the graphs are means±SEM. H2S, hydrogen sulfide; Na+/K+-ATPase inhibitor, a positive control; Oua, ouabain. See also Supplementary Figures S1 and S2.
H2S promotes Na+/K+-ATPase endocytosis in renal tubular epithelial cells
Na+/K+-ATPase endocytosis was examined by confocal microscopy. These results showed that Na+/K+-ATPase endocytosis was significantly increased in renal tubular epithelial cells treated with NaHS. This was apparent by the significant decrease in Na+/K+-ATPase staining on cell membranes at 60 min after adding NaHS at a concentration of 25 μM (Supplementary Fig. S2A). Cells treated with ouabain showed a more significant decrease in the membrane distribution of Na+/K+-ATPase (Supplementary Fig. S2A). These morphological observations were confirmed by determining Na+/K+-ATPase membrane-to-cytoplasm ratios by Western blot analysis, in which the membrane localization of Na+/K+-ATPase (including the α1 and β1 subunits) was significantly decreased in the presence of NaHS at 25 μM (Fig. 1E); whereas cytoplasmic Na+/K+-ATPase was increased (Fig. 1F). The Na+/K+-ATPase membrane-to-cytoplasm ratios showed that NaHS (25 μM) treatment caused ∼67% of Na+/K+-ATPase to be translocated from the cell membrane to the cytoplasm and that ouabain (50 nM) caused the translocation of ∼96% of membrane Na+/K+-ATPase compared with the vehicle-treated control (Fig. 1G).
Na+/K+-ATPase expression was not changed by NaHS treatment at concentrations ranging from 10 to 200 μM at 60 min after treatment (Fig. 1H, I). In addition, there was no change at times ranging from 5 to 120 min when used at a concentration of 25 μM (Fig. 1H, I). This showed that both H2S and ouabain caused a decrease in membrane Na+/K+-ATPase by promoting Na+/K+-ATPase endocytosis, not by inhibiting its overall expression.
H2S increases PI3K and Akt phosphorylation in renal tubular epithelial cells
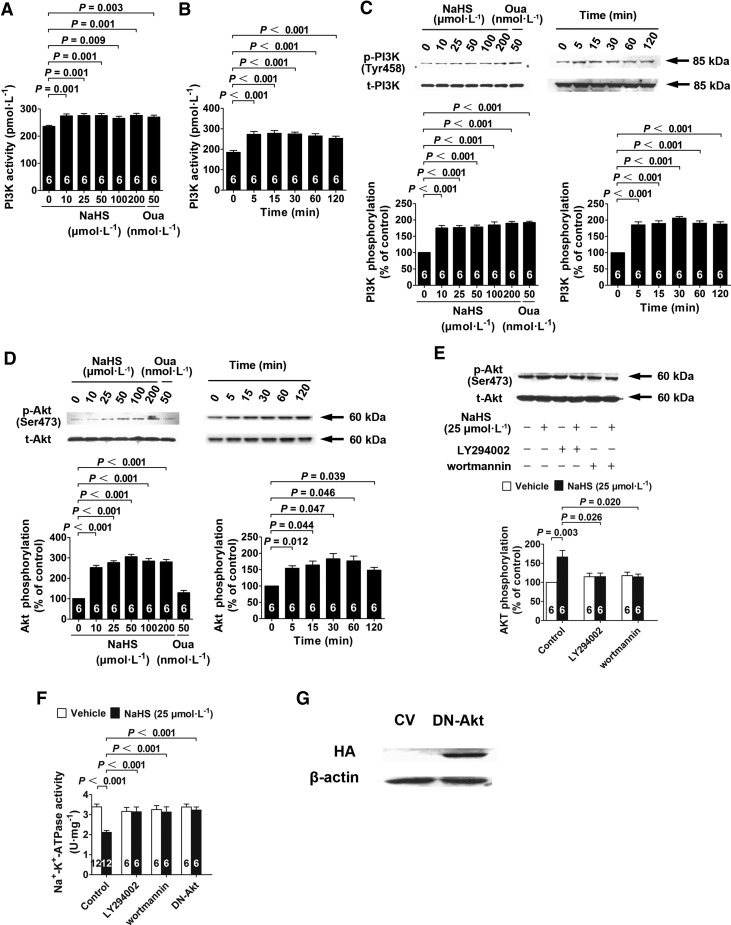
PI3K activity in renal tubular epithelial cells was increased with NaHS treatment at concentrations ranging from 10 to 200 μM at 60 min after treatment, and ouabain treatment exhibited a comparable effect when used at a concentration of 50 nM (Fig. 2A). This effect was first observed at 5 min and lasted until 120 min after adding NaHS at a concentration of 25 μM (Fig. 2B). NaHS treatment also increased PI3K phosphorylation at a similar concentration; this increase was time dependent (Fig. 2C).
FIG. 2.
H2S-induced Na+/K+-ATPase activity inhibition depends on PI3K and Akt. (A, B) Cells were treated with different concentrations of NaHS for 60 min (A) and with 25 μM NaHS for different durations (B) of increased PI3K activity. (C, D) Cells were treated with different concentrations of NaHS for 60 min and with 25 μM NaHS for different durations increased PI3K (C) and Akt (D) phosphorylation by Western blot analysis. (E) NaHS-induced Akt phosphorylation was abolished by a PI3K inhibitor with either LY294002 or wortmannin. (F) NaHS-induced Na+/K+-ATPase activity inhibition was abolished by either PI3K inhibitors (LY294002 and wortmannin) or transfection with DN-Akt. (G) Significant HA expression was detected in the cells transfected with DN-Akt after 24 h, suggesting a successful transfection and expression of DN-Akt. Data in the graphs are means±SEM. CV, control vector; DN-Akt, dominant-negative mutant of Akt; HA, hemagglutinin.
Akt phosphorylation in renal tubular epithelial cells was also increased after NaHS treatment at concentrations ranging from 10 to 200 μM at 60 min after treatment, whereas ouabain treatment did not show any significant effect on Akt phosphorylation when used at a concentration of 50 nM (Fig. 2D). The increase in Akt phosphorylation was first observed at 5 min and lasted until 120 min after NaHS treatment (Fig. 2D).
H2S-induced Na+/K+-ATPase endocytosis and activity inhibition depend on PI3K and Akt
Pretreatment of renal tubular epithelial cells with the PI3K inhibitors LY294002 (5 μM) and wortmannin (25 nM) inhibited the H2S-induced increase in Akt phosphorylation (Fig. 2E). Moreover, H2S-induced inhibition of Na+/K+-ATPase activity was abolished in renal tubular epithelial cells pretreated with LY294002 (5 μM) and wortmannin (25 nM; Fig. 2F). This H2S effect of inhibiting Na+/K+-ATPase activity was also abolished in cells transfected with a dominant-negative (DN) Akt mutant (DN-Akt; Fig. 2F). Successful transfection and expression of DN-Akt were confirmed by Western blot analysis using a hemagglutinin (HA)-tag conjugated with this mutant (Fig. 2G). This showed that H2S inhibited Na+/K+-ATPase activity by activating PI3K/Akt signals in renal tubular epithelial cells.
Interestingly, H2S-induced Na+/K+-ATPase endocytosis, which was characterized by decreased Na+/K+-ATPase on cell membranes, as observed by confocal microscopy, was also prevented by treatment with the PI3K inhibitors (LY294002 and wortmannin) and DN-Akt transfection (Supplementary Fig. S3A). The effects of H2S on increased Na+/K+-ATPase endocytosis at a concentration of 25 μM NaHS was abrogated by both PI3K inhibitors (LY294002 and wortmannin) and DN-Akt transfection (Fig. 3A). This indicated that H2S-induced Na+/K+-ATPase endocytosis in renal tubular epithelial cells was also dependent on PI3K/Akt signals.
FIG. 3.
H2S-induced Na+/K+-ATPase endocytosis depends on PI3K, Akt, gab1, and EGFR but not on c-Src. (A, B) Isolated cell membrane and cytoplasm through labeling of cell surface by biotinylation and, respectively, detected the expressions of Na+/K+-ATPase α1 and β1 subunits by Western blot analysis. NaHS-induced Na+/K+-ATPase endocytosis was abrogated by LY294002 (A), wortmannin (A), transfection with DN-Akt (A), and siRNAs knockdown of gab1 (B) and EGFR (B) but not c-Src (B). Data in the graphs are means±SEM. See Supplementary Figures S3, S4, and S6 for more information. EGFR, epidermal growth factor receptor; siRNA, small interfering RNA.
H2S-induced Na+/K+-ATPase endocytosis and activity inhibition depend on gab1, but not on c-Src
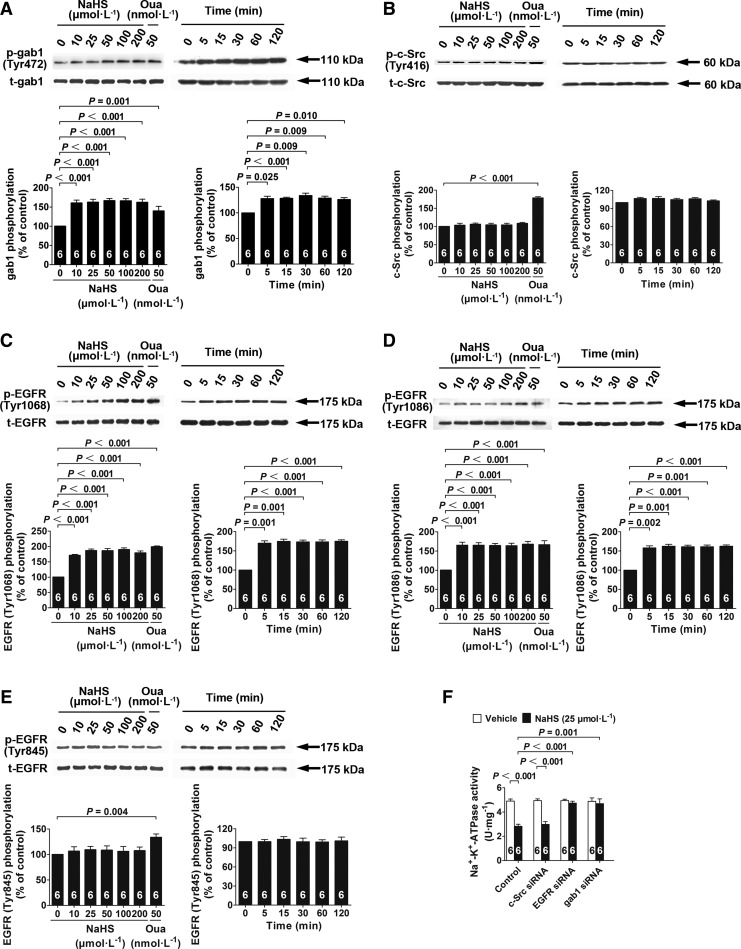
NaHS treatment resulted in increased gab1 phosphorylation in renal tubular epithelial cells when used at concentrations of 10, 25, 50, 100, and 200 μM for 60 min (Fig. 4A). This effect was first observed at 5 min and lasted until 120 min after NaHS treatment at 25 μM (Fig. 4A). Na+/K+-ATPase inhibition induced by NaHS (25 μM) treatment was abrogated by small interfering RNA (siRNA) knockdown of gab1 in renal tubular epithelial cells (Fig. 4F). The effects of NaHS treatment (25 μM) for promoting Na+/K+-ATPase endocytosis was also abrogated by siRNA knockdown of gab1, as demonstrated by a morphological examination of the cellular distribution of Na+/K+-ATPase in the membrane and cytoplasm (Supplementary Fig. S3A) and by Western blot analysis of the Na+/K+-ATPase membrane-to-cytoplasm ratios (Fig. 3B).
FIG. 4.
H2S-induced Na+/K+-ATPase activity inhibition depends on gab1 and EGFR but not on c-Src. (A–E) Cells were treated with different concentrations of NaHS for 60 min and with 25 μM NaHS for different durations; the phosphorylation of gab1 (A), c-Src (B), EGFR Tyr1068 (C), EGFR Tyr 1086 (D), and EGFR Tyr845 (E) was detected by Western blot analysis. (F) NaHS-induced Na+/K+-ATPase inhibition was abrogated by siRNAs knockdown of gab1and EGFR but not c-Src. Data in the graphs are means±SEM.
In contrast, c-Src phosphorylation was not changed in renal tubular epithelial cells treated with NaHS for 60 min at concentrations of 10, 25, 50, 100, and 200 μM; whereas ouabain (50 nM) treatment caused a significant increase in c-Src phosphorylation (Fig. 4B). Similarly, NaHS treatment (25 μM) had no effect on c-Src phosphorylation at 5, 15, 30, 60, and 120 min (Fig. 4B). Moreover, siRNA knockdown of c-Src did not alter the effects of NaHS (25 μM) to inhibit Na+/K+-ATPase activity (Fig. 4F) or the effects of NaHS (25 μM) to promote Na+/K+-ATPase endocytosis (Supplementary Fig. S3A, B).
H2S-induced Na+/K+-ATPase endocytosis and activity inhibition depend on EGFR
NaHS treatment for 60 min increased EGFR phosphorylation at two tyrosine sites, Tyr1068 (Fig. 4C) and Tyr1086 (Fig. 4D), but not at Tyr845 (Fig. 4E), when used at concentrations of 10, 25, 50, 100, and 200 μM. In comparison, ouabain treatment (50 nM) increased phosphorylation at all three of these tyrosine sites (Fig. 4C–E). The increase in EGFR phosphorylation at Tyr1068 and Tyr1086, but not at Tyr845, was first observed at 5 min after NaHS treatment (25 μM) and lasted until 120 min (Fig. 4C–E). In renal tubular epithelial cells with siRNA knockdown of EGFR, NaHS treatment (25 μM) for 60 min did not inhibit Na+/K+-ATPase activity (Fig. 4F). Similarly, NaHS treatment (25 μM) did not promote Na+/K+-ATPase endocytosis in cells with siRNA knockdown of EGFR, as demonstrated by a morphological examination of the cellular distribution of Na+/K+-ATPase in the membrane and cytoplasm (Supplementary Fig. S3A) and by Western blot analysis of the Na+/K+-ATPase membrane-to-cytoplasm ratios (Fig. 3B).
The efficiency of vector transfection in these knockdown experiments was>80% (Supplementary Fig. S4A). The efficiency of siRNA knockdown of c-Src, EGFR, and gab1 at the protein (Supplementary Fig. S4B) and mRNA level (Supplementary Fig. S4C) was ∼50% at 36 h after RNA interference.
EGFR and gab1, but not c-Src, are signaling elements upstream of PI3K and Akt for mediating the effects of H2S
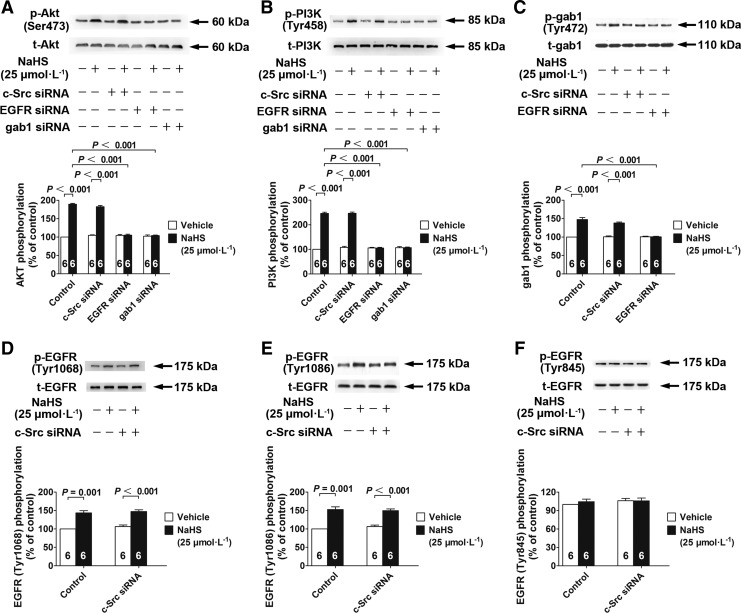
In renal tubular epithelial cells with siRNA knockdown of EGFR and gab1, NaHS treatment (25 μM) did not increase the phosphorylation of Akt or PI3K (Fig. 5A, B). In contrast, NaHS treatment (25 μM) increased Akt (Fig. 5A) and PI3K (Fig. 5B) phosphorylation in cells with siRNA knockdown of c-Src.
FIG. 5.
EGFR/gab1 but not c-Src are signaling elements upstream of PI3K/Akt for mediating the effects of H2S. (A, B) NaHS-induced Akt (A) and PI3K (B) phosphorylation was abrogated by siRNAs knockdown of gab1 and EGFR but not c-Src. (C) NaHS-induced gab1 phosphorylation was abrogated by siRNA knockdown of EGFR but not c-Src. (D–F) The siRNA knockdown of c-Src had no effect on the phosphorylation of EGFR Tyr1068 (D), Tyr1086 (E), or Tyr845 (F). Data in the graphs are means±SEM.
EGFR, but not c-Src, is a signaling element upstream of gab1 for mediating the effects of H2S
The increase in gab1 phosphorylation induced by NaHS treatment (25 μM) was abrogated by siRNA knockdown of EGFR, but not of c-Src (Fig. 5C). This suggested that EGFR was an upstream regulator of gab1 for mediating the effects of H2S.
In comparison, NaHS treatment (25 μM) increased EGFR phosphorylation at Tyr1068 and Tyr1086, and these effects were not abrogated by siRNA knockdown of c-Src (Fig. 5D, E). NaHS treatment (25 μM) did not alter EGFR phosphorylation at Tyr845 in cells transfected with control vectors or siRNA for c-Src (Fig. 5F). These results suggested that c-Src was not involved in the H2S/EGFR/gab1/PI3K/Akt pathway.
H2S directly interacts with and activates EGFR
Pure recombinant EGFR proteins were reacted with increasing concentrations of NaHS (10, 25, 50, 100, and 200 μM) for 60 min. NaHS treatment resulted in increased EGFR activity when used at concentrations of 50 and 100 μM compared with vehicle treatment (Fig. 6A). This suggested that H2S could directly react with EGFR proteins in the absence of other cellular components.
FIG. 6.
H2S directly activates EGFR and breaks its disulfide bond. (A) EGFR recombinant protein was treated with increasing concentrations of NaHS for 60 min, and the EGFR activity was detected. (B–E) NaH35S incubated with EGFR for different time scales (0–90 min) at 37°C (B) (n=6). Homologous competition curve for NaH35S binding against EGFR in vitro. Data were transformed into percentage of specific binding (%) and described by the one-site model (C) (n=6). A saturation binding experiment was performed at 37°C using increasing concentrations (0–12.8 μM) of NaH35S (D) (n=6). Scatchard analysis from saturation experiment gave the Kd and Bmax (E) (n=6). Peptides were used as a negative control. (F) Pretreated with NEM for 2 h to cover all -SH groups of the Cys residues in the intracellular kinase domain of EGFR. Cy3 and Cy5 labeled EGFR with fluorophore-conjugated maleimide. A representative fluorescence image was shown. The fluorescence intensities were quantified and normalized to intensities of the corresponding EGFR bands. (G) Differential Cys labeling of the recombination EGFR proteins treated with DTT, ddH2O, and NaHS, as done in (F). (H) Differential Cys labeling of the EGFR immunoprecipitated from cell lysate treated with DTT, ddH2O, and NaHS, as done in (F). (I–K) Mass spectrometry spectrum showing cleavage of the disulfide bond in Cys–Cys with treatment of NaHS and GSH. An S-sulfhydrated intermediate (Cys–SH) was identified during the process of the cleavage of the disulfide bond. Data in the graphs are means±SEM. Cys, cysteine; GSH, glutathione; NEM, N-ethylmaleimide. See Supplementary Figures S7 and S8 for more information.
To further examine the direct interaction between H2S and EGFR, binding assays were performed under physiological conditions (pH 7.4; 37°C). As shown in Figure 6B, specific binding between H2S and EGFR increased from 0 to 60 min in a time-dependent manner and decreased at 90 min. Therefore, incubation for 60 min was used for the following experiments.
In a homologous competition experiment, EGFR was incubated with NaH35S in the presence of increasing concentrations (10−8–10−1 M) of unlabeled NaHS. These results showed that NaHS inhibited the binding affinity of NaH35S in a concentration-dependent manner, and these data could be fit to a single binding site model (Fig. 6C). For a saturation-binding experiment, varying concentrations of NaH35S (0–12.8 μM) were incubated with EGFR, and the binding affinity was found to be saturable (Fig. 6D). Scatchard plot analysis (Fig. 6E) showed a single class of binding sites, with an equilibrium dissociation constant (Kd) of 1.399±0.933 nM and a maximum binding capacity (Bmax) of 1903±371.8 pmol·mg−1. In addition, the binding affinity reached a maximum when the molecular ratio of H2S to EGFR was ∼1:6 in solution, suggesting that the maximum binding ratio or capacity was at 1:6 between this ligand-receptor pair.
In all these experiments, synthesized peptides without any disulfide bonds were used as negative controls and showed no specific binding with NaH35S.
H2S breaks the disulfide bonds in the EGFR intracellular kinase domain
To further elucidate the molecular mechanism underlying H2S-induced EGFR activation, we modified the method used for fluorescent labeling of reactive cysteine (Cys) thiols. N-ethylmaleimide (NEM), an organic compound that reacts with thiols, was used to cover all -SH groups of the Cys residues in the intracellular kinase domain of EGFR. These results showed that Cy3 maleimide (Cy3) and Cy5 maleimide (Cy5) labeling was not detected with pure recombinant EGFR proteins that were pretreated with NEM (Fig. 6F). Therefore, NEM had completely covered all -SH groups of the Cys residues in the EGFR intracellular kinase domain, and this coverage was not washed away during this assay. This provided a clear background for the reliable detection of any new -SH groups that resulted from the cleavage of any disulfide bonds using Cy5 labeling.
As shown in Figure 6G, Cy3 labeling was not detected in all groups; this indicated that all -SH groups of the Cys residues in the EGFR intracellular kinase domain were completely covered and would not interfere with the detection of any new -SH groups which may be released after the cleavage of disulfide bonds. DTT treatment resulted in new -SH groups, as shown by Cy5 labeling, and indicated the cleavage of some disulfide bonds. This demonstrated that the EGFR intracellular kinase domain contained disulfide bonds in its structure. NaHS treatment also released new -SH groups, as shown by Cy5 labeling. This demonstrated that H2S broke these disulfide bonds in EGFR. A similar fluorescence assay was performed using EGFR proteins that were immunoprecipitated from cell lysates after treatment. These results were consistent with the experiment that used recombinant EGFR proteins. NaHS treatment also released new -SH groups, as shown by Cy5 labeling (Fig. 6H). This demonstrated that H2S broke these disulfide bonds in EGFR in cells.
H2S cleaves an S-S bond by a two-step mechanism with an S-sulfhydrated intermediate
Figures 6I and J show the mass spectrometry spectrum of the [M-H]− m/z 239.0 ion corresponding to Cys–Cys in the absence of NaHS and the mass spectrometry spectrum of the [M-H]− m/z 120.0 ion corresponding to Cys in the presence of NaHS. These data showed that H2S cleaved Cys–Cys into Cys. Moreover, the mass spectrometry spectrum of the [M-H]− m/z 152.0 ion corresponding to S-sulfhydrated Cys (C3H6NO2–S−+HS–S–C3H6NO2, see the following reactions) was also observed when the disulfide bond was cleaved in the presence of NaHS. This was in line with a two-step reaction for H2S cleavage of a disulfide bond (30):
Reaction 1: HS−+C3H6NO2–S–S–C3H6NO2→C3H6NO2–S−+HS–S–C3H6NO2
Reaction 2: HS−+HS–S–C3H6NO2→C3H6NO2–S−+HS–SH
Two HS− anions (derived from H2S) were required to cleave one disulfide bond. The first HS− bound with one of the sulfur atoms of the disulfide bond was under attack after the disulfide bond was cleaved. Then, the second HS− cleaved the first HS−, which was bound during the first reaction step, and formed the product HS–SH. This indicated that some HS− anions transiently bound with the sulfur atoms of the disulfide bond under attack.
In addition, mass spectrometry analysis showed that NaHS cleaved this disulfide bond at a concentration of 100 μM, whereas a much higher concentration of 10 mM was required for glutathione (GSH) to cleave this disulfide bond (Fig. 6K).
EGFR Cys797 (human) or Cys798 (rat) is required for H2S to activate EGFR and induce Na+/K+-ATPase endocytosis
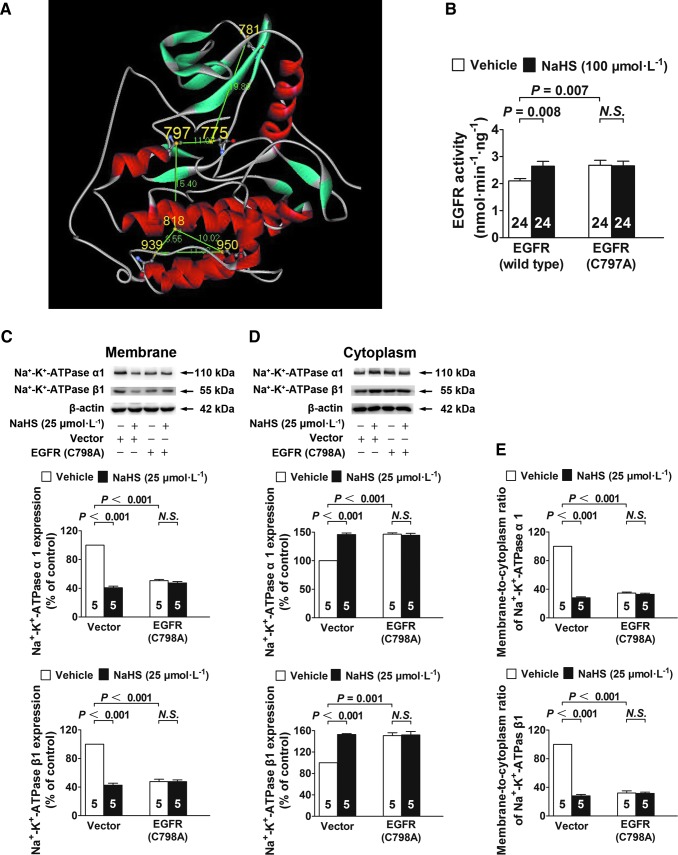
According to the crystal structure of human EGFR (PDB id: 2GS2) shown in Figure 7A, there are six Cys residues in its intracellular kinase domain. Of these, Cys797 is located in the ATP-binding pocket and is conserved among nine additional receptor and nonreceptor tyrosine kinases, including HER2, HER4, BLK, BMX, BTK, ITK, JAK3, TEC, and TXK (32). Given its location in the critical ATP-binding pocket and its conservation in various tyrosine kinases, we next focused on this residue. In addition, an analysis using WebLab ViewerPro software showed that Cys797 may form a disulfide bond with Cys775. On the basis of this theoretical analysis, Cys797 was mutated to an alanine and yielded a mutant human EGFR (C797A) protein.
FIG. 7.
EGFR Cys797 (human) or Cys798 (rat) is required for H2S to activate EGFR and induce Na+/K+-ATPase endocytosis. (A) The crystal structure of EGFR shows that Cys797 has a potential to form a disulfide bond with the Cys775. (B) A single amino-acid mutation at Cys797 caused a significant increase in EGFR kinase activity. H2S activates the wild-type EGFR but not the mutant EGFR (C797A). (C–E) Na+/K+-ATPase endocytosis was significantly increased in rat renal tubular epithelial cells transfected with the vectors expressing the mutant EGFR (C798A) as compared with the cells transfected with CVs. H2S treatment increased endocytosis of Na+/K+-ATPase in rat renal tubular epithelial cells transfected with CV, but not in the cells expressing the mutant EGFR. Data in the graphs are means±SEM.
This mutation caused a significant increase in EGFR kinase activity. Moreover, H2S no longer activated this mutant EGFR (C797A), although wild-type EGFR was activated (Fig. 7B). To further examine the function of this Cys residue in renal tubular epithelial cells (isolated from rats), we also constructed a rat mutant, EGFR (C798A), for which Cys798 was equivalent to Cys797 in human EGFR (the kinase core of EGFR is highly conserved between rats and humans). These results showed that Na+/K+-ATPase endocytosis was significantly increased in rat renal tubular epithelial cells transfected with lentivirus vectors which expressed EGFR (C798A) compared with that in cells transfected with control vectors. However, H2S treatment no longer increased Na+/K+-ATPase endocytosis in rat renal tubular epithelial cells that expressed mutant EGFR (C798A), although H2S treatment still increased Na+/K+-ATPase endocytosis in cells transfected with control vectors (Fig. 7C–E).
H2S/EGFR/gab1/PI3K/Akt signaling pathway components are activated in the kidney of chronic salt-loaded rats chronically treated with NaHS
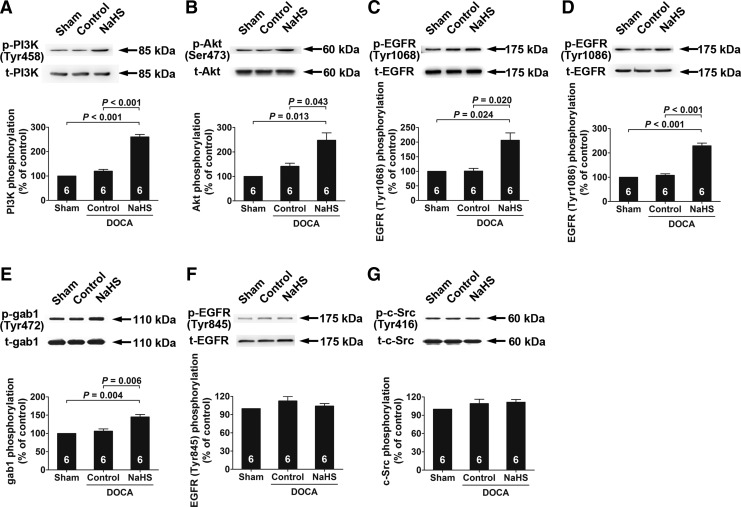
In the kidneys of deoxycorticosterone acetate (DOCA)-salt hypertensive rats treated with NaHS at a dose of 10 μmol·kg−1·day−1, the phosphorylation levels of PI3K, Akt, EGFR (at Tyr1068 and Tyr1086), and gab1 were increased (Fig. 8A–E). However, NaHS treatment did not alter EGFR phosphorylation at Tyr845 (Fig. 8F) or c-Src phosphorylation (Fig. 8G).
FIG. 8.
H2S/EGFR/gab1/PI3K/Akt signaling pathway components are activated in the kidney of chronic salt-loaded rats chronically treated with NaHS. (A–G) The phosphorylation levels of PI3K (A), Akt (B), EGFR Tyr1068 (C), EGFR Tyr1086 (D), gab1 (E), EGFR Tyr845 (F), and c-Src (G) in the kidney of chronic salt-loaded rats were detected by Western blot analysis. Data in the graphs are means±SEM. DOCA, deoxycorticosterone acetate.
H2S inhibits Na+/K+-ATPase activity and increases renal water and sodium excretion in chronic salt-loaded rats
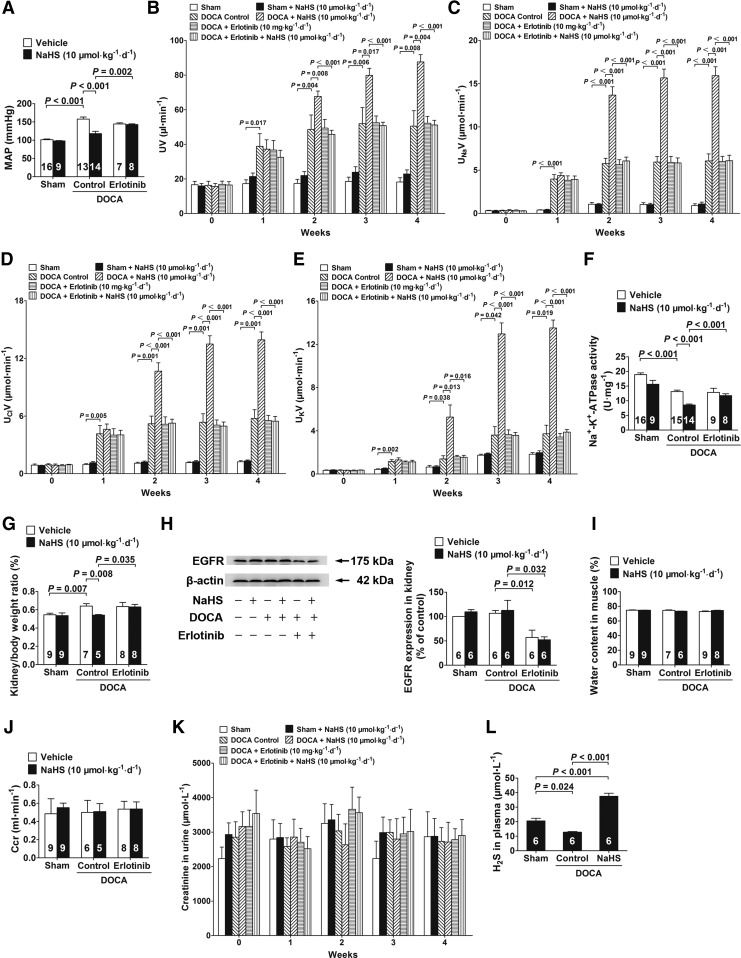
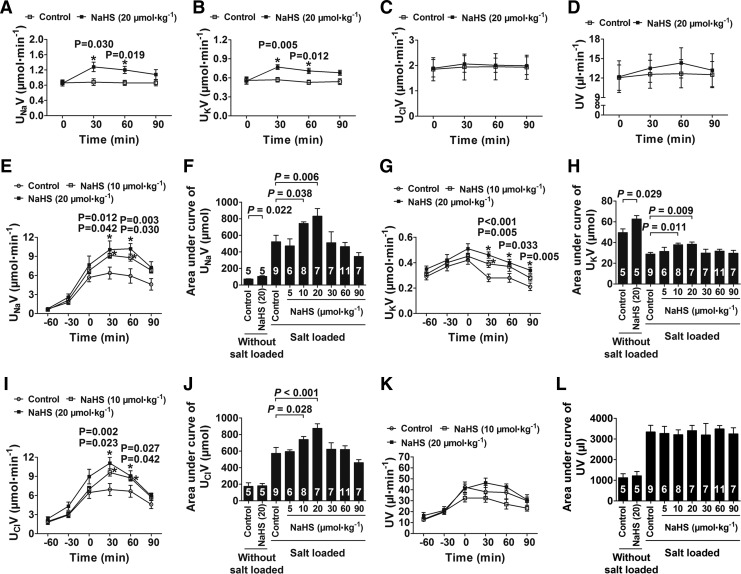
DOCA-salt hypertensive rats had significantly increased blood pressure (Fig. 9A). However, NaHS treatment (10 μmol·kg−1·day−1) significantly decreased the mean arterial pressure (MAP) in conscious rats (Fig. 9A) compared with that in control rats at week 4. This H2S effect for decreasing MAP was abrogated by treating these rats with the EGFR inhibitor erlotinib (Fig. 9A). Chronic treatment with NaHS also caused an increase in the urinary flow rate (UV; Fig. 9B), urinary sodium excretion (UNaV; Fig. 9C), urinary chlorine excretion (UClV; Fig. 9D), and urinary potassium excretion (UKV; Fig. 9E) during week 2 to 4 when administered at a dose of 10 μmol·kg−1·day−1. These H2S effects were also abrogated by treatment with erlotinib (Fig. 9B–E).
FIG. 9.
H2S inhibits Na+/K+-ATPase activity and increases renal water and sodium excretion in chronic salt-loaded rats. (A) MAP in conscious rats in a chronic salt-loaded experiment at week 4. (B) The UV was measured in the period of a chronic salt-loaded experiment. (C–E) In the chronic salt-loaded experiment, the UNaV (C), UClV (D), and UKV (E) were measured. (F) Kidneys were removed and isolated to renal tubular epithelial cells to determinate Na+/K+-ATPase enzymatic activity. (G) The kidney/body weight ratio in chronic salt loaded experiment at week 4. The kidney/body weight ratio=kidney weight/body weight×100%. (H) EGFR protein levels in kidneys were decreased by ∼50% in the rats treated with erlotinib for 4 weeks by western blot analysis. (I–K) Water content in muscle (I), Ccr (J), and urine creatinine (K) were examined in the chronic salt-loaded experiment at week 4. (L) Plasma H2S concentration was tested in the chronic salt-loaded experiment at week 4. Data in the graphs are means±SEM. Ccr, creatinine clearance rate; erlotinib, an EGFR inhibitor; MAP, mean arterial pressure; UClV, urinary chlorine excretion; UKV, urinary potassium excretion; UNaV, urinary sodium excretion; UV, urinary flow rate. See Supplementary Figure S9 for more information.
Moreover, Na+/K+-ATPase enzymatic activity was decreased in renal tubular epithelial cells isolated from DOCA-salt hypertensive rats that were chronically treated with NaHS at a dose of 10 μmol·kg−1·day−1 compared with that in renal tubular epithelial cells isolated from vehicle-treated DOCA-salt hypertensive rats at week 4 (Fig. 9F). Similarly, this H2S effect for inhibiting Na+/K+-ATPase activity was abrogated by treatment with erlotinib (Fig. 9F). In addition, NaHS treatment (10 μmol·kg−1·day−1) caused a significant decrease in the kidney/body weight ratio (Fig. 9G) compared with vehicle treatment at week 4, and this H2S effect was also abrogated by treatment with erlotinib (Fig. 9G).
EGFR protein levels in kidneys were decreased by ∼50% in the rats treated with erlotinib for 4 weeks (Fig. 9H). In addition, NaHS treatment (10 μmol·kg−1·day−1) had no effect on the water content in muscles (Fig. 9I), the creatinine clearance rate (Ccr; Fig. 9J), or urine creatinine levels (Fig. 9K) in chronic salt-loaded rats. Similarly, NaHS treatment (10 μmol·kg−1·day−1) had no effect on biochemical profiles at week 4, including glutamic–pyruvic transaminase, creatinine, glucose, triglyceride, and total cholesterol levels (Supplementary Table S1). Moreover, H2S plasma concentrations in DOCA-salt hypertensive rats (12.78±0.44 μM) were significantly decreased compared with those in sham-operated rats (20.53±1.88 μM) at week 4. NaHS treatment (10 μmol·kg−1·day−1) by an intraperitoneal injection significantly increased plasma H2S concentrations to 37.47±2.13 μM (Fig. 9L).
H2S increases water and sodium excretion in the kidneys of rats with or without acute salt loading
An intravenous injection of NaHS bolus caused a significant decrease in blood pressure and renal blood flow (RBF) (Supplementary Fig. S5A–C), while a 1-min continuous infusion of NaHS at a dose of 20 μmol·kg−1 in rats without salt loading did not change MAP or RBF (Supplementary Fig. S5D–F). Continuous infusion with NaHS for 1-min at doses ranging from 5 to 90 μmol·kg−1 in acute salt-loaded rats also did not cause any significant changes in MAP (Supplementary Fig. S5G, H), RBF (Supplementary Fig. S5I, J), or glomerular filtration rate (GFR) (Supplementary Fig. S5K, L). Therefore, a 1-min continuous infusion of NaHS was used for the following acute in vivo experiments to examine the possible direct effects of H2S on renal sodium excretion, independent of renal hemodynamics.
After a 1-min infusion of NaHS at a dose of 20 μmol·kg−1 in rats without salt loading, there was a significant increase at 30 and 60 min in UNaV (Fig. 10A) and UKV (Fig. 10B), but not in UClV (Fig. 10C) or UV (Fig. 10D), compared with those in vehicle-treated rats.
FIG. 10.
H2S increases water and sodium excretion in the kidneys of rats with or without acute salt loading. (A–D) NaHS treatment (20 μmol·kg−1) caused a significant increase in UNaV (A) and UKV (B), but not in UClV (C) or UV (D) at 30 and 60 min after treatment in rats without salt loading (n=5). (E–L) In acute salt-loaded rats, NaHS treatment (10 and 20 μmol·kg−1) increased UNaV (E), UKV (G), and UClV (I) but not UV (K). The areas under curve of UNaV (F), UKV (H), UClV (J), and UV (L) were also shown (n=6–11). Data in the graphs are means±SEM. See Supplementary Figure S5 for more information.
In acute salt-loaded rats, blood pressure significantly increased after salt infusion for 1 h, and this increase lasted for 90 min. For rats with acute salt loading, a 1-min infusion of NaHS at doses of 10 and 20 μmol·kg−1 caused an increase at 30 and 60 min in UNaV (Fig. 10E, F), UKV (Fig. 10G, H), and UClV (Fig. 10I, J). However, a 1-min infusion of NaHS at doses ranging from 5 to 90 μmol·kg−1 did not cause any significant changes in UV (Fig. 10K, L).
Discussion
Currently, there is no information regarding a possible role of H2S in the regulation of endocytosis of important membrane proteins that control transmembrane transport of various agents, which is one of the fundamental biological processes of cells. Here, we showed that H2S promoted Na+/K+-ATPase endocytosis in renal tubular epithelial cells, thereby inhibiting sodium absorption into cells.
Na+/K+-ATPase endocytosis is regulated by endogenous ouabain (16, 19). After binding with Na+/K+-ATPase, ouabain activates c-Src, which subsequently activates EGFR. Via the adaptor protein gab1, EGFR binds and activates PI3K, which then forms the Na+/K+-ATPase/c-Src/EGFR/PI3K complex. As a consequence, PI3K recruits AP2 to bind with Na+/K+-ATPase and activates clathrin to induce the endocytosis of Na+/K+-ATPase (17, 18). However, we found that H2S not only increased PI3K phosphorylation but also increased Akt phosphorylation, and the effect of H2S to induce Na+/K+-ATPase endocytosis was abrogated by the expression of DN-Akt. This illustrated that Akt played a pivotal role in mediating H2S signals. This is in contrast to ouabain-induced Na+/K+-ATPase endocytosis, in which Akt is not involved (17, 18). However, PI3K seems to be required for both H2S and ouabain, as PI3K has been shown to mediate ouabain signals during Na+/K+-ATPase endocytosis (17, 39) in renal tubular epithelial cells. In the present study, H2S also activated PI3K, and treatment with PI3K inhibitors abrogated PI3K phosphorylation and Na+/K+-ATPase endocytosis induced by H2S. These data suggested that H2S signals were transduced to Akt via PI3K. This proposition was further supported by the increased phosphorylation of PI3K and Akt observed in the kidneys of salt-loaded rats treated with NaHS. Interestingly, PI3K/Akt signals have been shown to play a pivotal role in mediating the effects of H2S for promoting angiogenesis (4) and protecting the myocardium against ischemia/reperfusion injury (31). Therefore, it is possible that PI3K/Akt may be a common pathway to transduce H2S signals in various cell types to mediate multiple H2S functions in different cells/tissues.
Various signaling elements, including cAMP/PKA (1), PKC (6), and cGMP/PKG (21), have been shown to play important roles in the regulation of Na+/K+-ATPase activities in renal tubular epithelial cells. The results of the present study showed that H2S had no effect on cAMP, cGMP, PKA, and PKC levels and the phosphorylation of PKA and PKC. These results suggest that the effects of H2S on Na+/K+-ATPase endocytosis may not depend on the cAMP/PKA, PKC, and cGMP/PKG pathways.
The next question was regarding the receptor for H2S to induce Na+/K+-ATPase endocytosis. Endogenous ouabain binds to ouabain-binding sites on Na+/K+-ATPase, which consequently activates c-Src, EGFR, and PI3K to generate a Na+/K+-ATPase/c-Src/EGFR/PI3K complex (17, 39). This complex recruits AP2 to bind with the α subunit of Na+/K+-ATPase and causes Na+/K+-ATPase endocytosis (37). Since PI3K was involved in both the H2S and ouabain signaling pathways, we hypothesized that H2S and ouabain may share some common signaling pathways for inducing Na+/K+-ATPase endocytosis. Interestingly, a comparison of the signaling elements involved in the H2S and ouabain signaling pathways revealed a difference in these two pathways; c-Src was required for the ouabain signaling pathway, but not for the H2S signaling pathway. In contrast, Akt was required for the H2S signaling pathway, but not for the ouabain signaling pathway. However, EGFR was activated in both pathways. This suggests that ouabain and H2S share some common pathways. We also found that H2S increased EGFR kinase activity in a cell-free system without any other cellular components. This suggested that H2S directly interacted with and activated EGFR.
EGFR is a member of the receptor tyrosine kinase family. After a ligand binds to its extracellular domain, EGFR monomers are dimerized and different tyrosine sites are phosphorylated by autotransphosphorylation. This results in the activation of a series of downstream signaling elements, including Ras, MAPK, PKC, and PI3K (20). Different tyrosine phosphorylation sites are shown to be involved in the transduction of these signals to several distinct pathways downstream of EGFR (20). For example, Tyr845 is shown to be involved in the c-Src kinase pathway (3), while Tyr1068 and Tyr1086 transduce signals to the PI3K/Akt and Ras/Raf/MAPK pathways. Interestingly, ouabain signals are associated with phosphorylation of all three tyrosine sites (Tyr845, Tyr1068, and Tyr1086) and the phosphorylation of c-Src; however, H2S increased the phosphorylation of Tyr1068 and Tyr1086, but not of Tyr845. Therefore, our data suggest that H2S activates EGFR by some mechanism(s) different from those used by ouabain.
In the present study, siRNA knockdown of EGFR abrogated H2S-induced Na+/K+-ATPase endocytosis and inactivation. It is shown that gab1 acts as an adaptor between EGFR and PI3K, thereby playing a pivotal role in transducing signals from EGFR to PI3K (25). Interestingly, H2S also increased gab1 phosphorylation in renal tubular epithelial cells. Moreover, siRNA knockdown of gab1 abrogated the effects of H2S for inducing Na+/K+-ATPase endocytosis and inactivation. This indicated that gab1 also played a central role in transducing H2S signals. A role for gab1 was further clarified in that siRNA knockdown of EGFR, but not of c-Src, was required for the transduction of H2S signals to gab1. These results revealed a new pathway for inducing Na+/K+-ATPase endocytosis and inactivation: the H2S/EGFR/gab1/PI3K/Akt pathway.
The typical ouabain-induced signaling pathway requires c-Src to transduce ouabain signals to EGFR, which results in Na+/K+-ATPase endocytosis and inactivation. In contrast, H2S appeared to skip some signaling pathways upstream of EGFR and directly targeted its receptor, EGFR. This proposition was further supported by a binding assay for EGFR using radiolabeled H2S. These results showed that binding was specific and reversible, although this binding was weak and transient. This may not have reflected typical ligand–receptor binding; rather, it reflected a process involving cleavage of disulfide bonds by H2S.
We previously showed that an HS− anion (derived from H2S) cleaved disulfide bonds. Two HS− anions were required to cleave one disulfide bond by a sulfur–sulfur nucleophilic attack mechanism in a two-step reaction (30). In the first step, the first HS− anion bound with one of the sulfur atoms of the disulfide bond to yield an S-sulfhydrated intermediate, while the original disulfide bond was cleaved. Then, the second HS− anion decreased the S-sulfhydrated-SH group, and the first HS− anion bound in the first step was finally cleaved off during the second step (30). By this mechanism, H2S did show some direct binding to its target molecule (i.e., formation of the transient S-sulfhydrated intermediate). This proposition was supported by mass spectrometry analysis in the present study, in which an S-sulfhydrated intermediate was identified during the cleavage of a disulfide bond by H2S.
However, the involvement of EGF, the classic EGFR ligand, in regulating Na+/K+-ATPase endocytosis remains unknown. In the present study, exogenous EGF had no effect on Na+/K+-ATPase endocytosis, although it attenuated the effects of H2S on Na+/K+-ATPase endocytosis. The mechanisms underlying this phenomenon remain unknown. It is possible that the conformation of the EGFR intracellular kinase domain is changed on extracellular EGF binding, which could result in an EGFR conformation which is less responsive to H2S.
The next question was regarding the mechanism by which H2S directly interacted with EGFR. We recently showed that H2S recognized one of the sulfur atoms of a disulfide bond in the intracellular kinase domain of VEGFR2 by a sulfur–sulfur nucleophilic attack on the intrinsic inhibitory Cys1024–Cys1045 disulfide bond. This resulted in a change in the conformation of the VEGFR2 kinase domain and relieved it from its intrinsic inhibitory status (30). It should be noted that both EGFR and VEGFR2 are receptor tyrosine kinases. The major structural difference between these two kinases lies in their extracellular domains, as they have similar intracellular kinase cores (15). In addition, the Cys1024–Cys1045 disulfide bond is in the VEGFR2 intracellular kinase core and acts as a molecular switch for H2S to regulate the structure and function of VEGFR2 (30). From this, we hypothesized that receptor tyrosine kinases may act as receptors for H2S for mediating the various biological effects of this gaseous transmitter.
The data from our present study support this idea by showing that another receptor tyrosine kinase, EGFR, acts as a receptor for H2S to regulate Na+/K+-ATPase endocytosis. EGFR may also contain an intrinsic inhibitory disulfide bond to act as a molecular switch for H2S to mediate its initial actions on EGFR and the subsequent effects on Na+/K+-ATPase endocytosis and inactivation. This was further supported by our fluorescence labeling of reactive Cys thiols, in which disulfide bonds were identified in the EGFR intracellular kinase domain; H2S treatment cleaved these disulfide bonds in a cell-free system without any other cellular components. This suggests that the EGFR intracellular kinase domain also contains some disulfide bonds that are a target motif for H2S regulation. However, this method cannot be used to precisely identify the Cys sites that may form disulfide bonds.
Using a different method, we investigated the function of certain critical Cys residues located in the EGFR kinase core that may have been possible sites for forming disulfide bonds. We found that a particular Cys residue (Cys797 in human EGFR or Cys798 in rat EGFR) had intrinsic inhibitory activity in the regulation of EGFR kinase activity and Na+/K+-ATPase endocytosis in renal tubular epithelial cells. Moreover, a mutation at this Cys blocked the effects of H2S for activating EGFR and increasing Na+/K+-ATPase endocytosis. These data further indicate that this particular Cys residue acts as a molecular switch for H2S to regulate EGFR function. However, our current data are not sufficient to locate the other Cys residue that may form a disulfide bond with Cys797/Cys798.
Another member of the receptor tyrosine kinase family that is responsive to H2S is the insulin receptor. We previously found that H2S directly reacted with the insulin receptor and caused an increase in its kinase activity and subsequent glucose uptake (35). All of this evidence together supports our idea that the receptor tyrosine kinase family is an H2S-dependent protein kinase family. Although the extracellular receptor domains still bind to their cognate extracellular ligands, VEGF, EGF, and insulin, respectively, H2S controls the common intracellular kinase core, which is essential for the final activation of these receptor tyrosine kinases.
Kirley (13) showed that there were three disulfide bonds in the β subunit of Na+/K+-ATPase. This enzyme is inactivated in the presence of high concentrations of β-mercaptoethanol, which may break these disulfide bonds. In the present study, H2S directly reacted with Na+/K+-ATPase, which resulted in an increase in its activity. However, in live cells, H2S promoted Na+/K+-ATPase endocytosis, which, ultimately, resulted in the inhibition of Na+/K+-ATPase activity. This suggests that the effects of H2S on Na+/K+-ATPase are multifaceted and that the ultimate effects in a live cell or in the body are the results of interactions among several different pathways triggered by H2S.
Reactive oxygen species (ROS) may also be involved in these effects of H2S. For example, H2S protects the myocardium against ischemia/reperfusion by scavenging ROS (7). However, H2S treatment did not change ROS levels in renal tubular epithelial cells. This does not support the finding that the effects of H2S for inducing Na+/K+-ATPase endocytosis are mediated by the regulation of ROS levels in renal tubular epithelial cells.
It should be noted that there are other reducing factors such as GSH in cells which may also cleave disulfide bonds. However, H2S functions as a unique reducing factor by exhibiting some properties that are different from GSH. H2S is the smallest reducing factor identified till date. It may be able to penetrate into some deep niche within a large protein molecule, where a GSH molecule may be unable to penetrate because of its large size. Moreover, we recently showed that H2S cleaved disulfide bonds by some mechanism(s) other than its reducing capability (30). This may aid in explaining why H2S was more potent than GSH for cleaving disulfide bonds, as determined by mass spectrometry in the present study.
It is also possible that the distributions of H2S and GSH are different in different tissues and that they may be in different intracellular compartments within a single cell. If this is true, then H2S and GSH may target different molecules according to their tissue/intracellular distribution pattern. Although experimental efforts are limited because of current methodology limitations, this hypothesis warrants further investigation. Sensitive indicators which are specific for H2S and GSH that can be used in live cells or with in vivo models would be necessary for such experiments.
In addition, H2S treatment increased sodium excretion in both acute and chronic salt-loaded rats, and this effect was abrogated by cotreatment with an EGFR inhibitor. These in vivo data are in line with our in vitro findings in which H2S activated EGFR, promoted Na+/K+-ATPase endocytosis and inhibition, and resulted in decreased sodium uptake by renal tubular epithelial cells. The kidney is an important organ for blood pressure regulation (9). Indeed, mechanisms other than the currently accepted EGFR/gab1/PI3K/Akt pathway may be involved in the renal actions of H2S. We showed here that H2S also inhibited NHE3 activity in renal tubular epithelial cells; NHE3 is known to be involved in the regulation of renal function (14). In this context, other pathways such as NHE3 may also be involved in mediating the renal actions of H2S.
In particular, Na+/K+-ATPase is the most important transporter involved in the regulation of the water and sodium balance in renal tubular epithelial cells. Renal water and sodium homeostasis is determined by the GFR and the reabsorption of water and sodium in renal tubules. In the present study, H2S treatment induced an increase in sodium excretion that was not dependent on RBF or the GFR in the kidneys of salt-loaded rats. Moreover, plasma ouabain levels and ouabain expression in the kidney were not changed in rats treated with H2S. This excludes the possibility that H2S may promote sodium excretion by inducing an increase in ouabain biosynthesis. These in vivo data support our in vitro findings that H2S directly acts on renal tubular epithelial cells to regulate sodium transport. The in vivo H2S effects in these salt-loaded models may translate to the development of therapeutic approaches for diseases related to sodium retention.
Taken together, the results of the present study provide the first evidence that H2S can regulate the endocytosis of a functional membrane protein, Na+/K+-ATPase, in renal tubular epithelial cells. This H2S effect is mediated by the H2S/EGFR/gab1/PI3K/Akt pathway, among which EGFR acts as a receptor for H2S. EGFR Cys797 (human) or Cys798 (rat) residues play an essential role for H2S to activate EGFR and increase Na+/K+-ATPase endocytosis in renal tubular epithelial cells. These results reveal a new mechanism for renal sodium and water homeostasis in the kidney. In addition, other pathways such as NHE3 may be involved in mediating the renal effects of H2S. Moreover, the present data suggest the hypothesis that the receptor tyrosine kinase family is an H2S-dependent protein kinase family. This may aid in explaining the mechanisms that underlie the various biological effects of H2S in the vasculature, insulin-targeted tissues, and the kidney, in which H2S-dependent protein kinases have a common molecular switch for H2S regulation. In addition, this new H2S/EGFR/gab1/PI3K/Akt pathway may aid in the development of novel approaches for the regulation of renal sodium and water homeostasis for treating diseases related to renal sodium homeostasis dysfunction, such as hypertension.
Materials and Methods
The detailed methods used for immunofluorescence microscopy; Western blotting; real-time polymerase chain reaction (PCR); measurements of cGMP, cAMP, PKC, PKA, ouabain, ROS, and GSH; NHE3 activity; total antioxidant capacity (T-AOC); and a rat starvation protocol are described in Supplementary Data.
Primary culture of rat renal tubular epithelial cells
Before animal surgery, all glassware and surgical tools were sterilized in an autoclave. Male Sprague–Dawley (SD) rats weighing 50–80 g were used. Rats were anesthetized with chloral hydrate (300 mg·kg−1 body weight) and cleaned with 75% ethanol. The kidneys were excised, capsules were removed, and cortices were dissected out from each kidney. Cortical slices were minced and pressed through an 80-mesh (pore size: 180 μm) stainless steel screen while being rinsed with ice-cold phosphate-buffered saline (PBS) onto a 100-mesh (pore size: 150 μm) screen. Tissue on the 100-mesh screen was rinsed, transferred to 15-ml centrifuge tubes, and centrifuged for 5 min at 260 g. Then, 1.5 ml of 0.25% trypsin was added to the tissue pellet for digestion at 37°C for 20 min. After this, 3 ml of Dulbecco's modified Eagle's medium/Ham's nutrient mixture F12 (DMEM/F12) that contained 10% fetal bovine serum (FBS) was added to terminate digestion and centrifuged for 8 min at 260 g. The supernatant was removed. Finally, renal tubular segments were suspended in 3 ml of DMEM/F12 containing 10% FBS and cultured in 60-mm dishes at 37°C in a 5% CO2 incubator. Fresh medium was replaced after 72 h and every 48 h thereafter for the primary culture of rat renal tubular epithelial cells. Cells cultured for 6–7 days were identified by confocal microscopy and transmission electron microscopy.
Na+/K+-ATPase enzymatic activity
Renal tubular epithelial cells were pretreated with different inhibitors or vehicle (control) and stimulated with increasing doses of NaHS (0–200 μM) for 60 min or with 25 μM NaHS for different time periods (0–120 min), after which cell suspensions were harvested. A cell suspension was ground by ultrasonication, and the protein concentration was determined with a BCA reagent. Na+/K+-ATPase enzymatic activity was determined using the following reagents: (i) 30 mM imidazole-HCl, 130 mM NaCl, 20 mM KCl, 4 mM MgCl2 and (ii) 30 mM imidazole-HCl, 4 mM MgCl2, and 1 mM ouabain at a pH of 7.4. A reaction was initiated by adding 4 mM Tris-ATP and incubated at 37°C for 10 min. ATP enzymatic hydrolysis was terminated by adding 100 μl of 12.5% trichloroacetic acid, mixing by inversion, and centrifugation at 3500 g for 10 min to obtain a supernatant. Enzymatic activity was calculated as a function of liberated inorganic phosphate (Pi) on the basis of a color reaction. To estimate Pi release, 0.3 ml of a supernatant was added to 1 ml of a chromogenic reagent (10% ammonium molybdate in 10 N H2SO4 and 5% ferrous sulfate) and maintained at room temperature for 2 min. Then, 1 ml of chromogenic termination solution was added and maintained at room temperature for 5 min. Finally, A636 was determined with a microplate reader (Tecan Infinite M200, Männedorf, Switzerland). Na+/K+-ATPase activity was the difference in Pi contents between media (i) and (ii) and expressed as μmoLPi·mgprotein−1·h−1.
Renal tubular epithelial cells were stimulated with increasing doses of NaHS (0–200 μM) for 60 min, after which membrane and cytoplasmic fractions were isolated with a Proteo Extract Transmembrane Protein Extraction Kit (Millipore, Hayward, CA). Na+/K+-ATPase activity in these membrane and cytoplasmic fractions was determined as described earlier.
Cell surface biotinylation
Cell surface biotinylation was done as previously described (22). Briefly, renal tubular epithelial cells were starved for 6 h and pretreated with different inhibitors. Cells were then stimulated with 25 μM NaHS or vehicle for 60 min. After three washes with ice-cold PBS-Ca-Mg (PBS containing 100 μM CaCl2 and 1 mM MgCl2), surface proteins were biotinylated twice with EZ-Link Sulfo-NHS-SS-Biotin [1.5 mg·ml−1 in 10 mM triethanolamine (pH 9.0) containing 2 mM CaCl2 and 150 mM NaCl] for 25 min on ice. After biotin labeling, cells were scraped in IP buffer containing a protease inhibitor cocktail, frozen in liquid nitrogen, rapidly thawed, probe sonicated twice in an ice-water bath, and frozen thawed again. The cell suspension was centrifuged at 16100 g for 5 min at 4°C, and the supernatant was transferred to clean tubes. Biotinylated proteins were separated with a 50% slurry of ImmunoPure immobilized streptavidin-agarose beads overnight at 4°C with end-to-end rotation. After centrifugation, the supernatants were considered to include cytoplasmic proteins and transferred to another tube. Immunomagnetic beads were washed four times with IP buffer that contained 1% Triton and 0.1% sodium dodecyl sulfate (SDS) and once with 50 mM Tris-HCl (pH 7.4), and they were finally resuspended in Laemmli sample buffer. Western blot analysis using Na+/K+-ATPase α1, β1 subunit antibodies was performed as described in Supplementary Data.
PI3K measurements
Renal tubular epithelial cells were stimulated with increasing doses of NaHS (0–200 μM) for 60 min or 25 μM NaHS for different time periods (0–120 min). Cells were lysed with lysis buffer [20 mM Tris-HCl (pH 7.4), 137 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.1 mM sodium vanadate, 1% NP40, and 1 mM PMSF]. PI3K levels were determined with an enzyme immunoassay kit (Echelon Biosciences, Inc., Salt Lake City, UT) according to the manufacturer's instructions. These values were normalized by the cell lysate protein concentration.
Transfection of a DN-Akt mutant
A HA-tagged DN (kinase-inactive mutant Myr-Akt-K179M) Akt was a kind gift from Jin Q. Cheng (Department of Pathology and Interdisciplinary Oncology, University of South Florida College of Medicine; H. Lee Moffitt Cancer Center, Tampa, FL). A pcDNA3 vector that contained DN-Akt cDNA or its control vector was transfected into renal tubular epithelial cells using Lipofectamine 2000 and incubated for 24 h in DMEM/F12 in a 37°C incubator with 95% O2 and 5% CO2. HA expression detected by Western blot analysis verified successful transfection.
Transfection of siRNAs for c-Src, EGFR, and gab1
siRNAs for c-Src, EGFR, and gab1 were obtained from Ambion (Foster City, CA). These siRNAs or control siRNA were transfected into renal tubular epithelial cells using SiPORT NEOFX Transfection Agent and incubated for 36 h in DMEM/F12 with 10% FBS in a 37°C incubator with 95% O2 and 5% CO2. Protein expression and mRNA expression by Western blot analysis and real-time PCR verified successful transfection.
Recombinant EGFR kinase activity
The kinase activities of pure recombinant EGFR (Signalchem, Richmond, Canada) and mutant EGFR (C797A) were assessed in the presence or absence of NaHS in a cell-free system that contained no other intracellular signaling components. EGFR activity was assayed using an EGFR kinase assay kit (Promega, Madison, WI) according to the manufacturer's instructions.
Receptor binding assays
An Na235S (5 mCi·mmol−1) solution was adjusted to a pH of 8.66 to yield NaH35S just before a binding experiment. Binding assays were performed according to methods described by Czernik and Petrack (5), with modifications. Briefly, EGFR proteins (1.12 μM) were incubated in 400 μl of a medium that contained 50 mM of Tris-HCl buffer (pH 7.4) and 5 μM NaH35S. After incubation for different time periods (0–90 min) at 37°C, 100 μl of ice-cold NaCl was added to terminate the reaction. Bound and free ligands were separated using a Microcon YM-3 ultrafiltration centrifuge tube (Millipore, Boston, MA) at 12,000 g for 60 min. Radioactivity was measured in a scintillation cocktail (Packard Ultima GOLD; PerkinElmer, Wellesley, MA) using a liquid scintillation analyzer (Tri-Carb 2910TR; PerkinElmer) with 93% counting efficiency.
For a competition assay, increasing concentrations of unlabeled NaHS (10−8–10−1 M) were coincubated with 5 μM NaH35S at 37°C for 60 min. For a saturation binding assay, EGFR was reacted with increasing concentrations of NaH35S (0–12.8 μM) and incubated at 37°C for 60 min. Maximum binding (Bmax) and an equilibrium dissociation constant (Kd) were determined using linear regression analysis by the Scatchard method (26) with Prism software (GraphPad Instat, version 5.00). Nonspecific binding was determined from the amount of radioactivity bound in the absence of EGFR, which accounted for 20%–30% of the total bound radioactivity in all experiments. This nonspecific component was subtracted from the total bound radioactivity to obtain the corresponding specific binding.
Fluorescence labeling of reactive Cys thiols
Fluorescence labeling of reactive Cys thiols was performed as previously described (12). EGFR-recombinant proteins were subjected to immunoprecipitation with an EGFR antibody. These immunoprecipitates were incubated with 10 μM NEM at room temperature for 2 h and washed thrice with PBS (pH 7.4). Cy3 maleimide (100 μM) was added for 30 min, and the immunoprecipitates were washed. Then, the immunoprecipitates were treated with vehicle, DTT (25 mM), or NaHS (25 mM) at room temperature for 1 h and washed. The immunoprecipitates were relabeled with Cy5 maleimide (100 μM) at room temperature for 30 min and washed. The double-labeled immunoprecipitates were extracted with NP40, boiled in a 6×sample buffer, and separated on an SDS–polyacrylamide gel electrophoresis (PAGE). Fluorescent images were acquired with a Typhoon FLA9500 variable mode imager (GE Healthcare, Buckinghamshire, United Kingdom), and fluorescence levels were quantified using ImageQuant version 5.0 software (Molecular Dynamics).
Renal tubular epithelial cells were lysed with NP40, and the protein concentration was measured with a BCA reagent. Then, 200 μg of protein was used for immunoprecipitation with an EGFR antibody. These immunoprecipitates were incubated with 70 μM NEM at room temperature for 2 h and washed thrice with PBS (pH 7.4). Fluorescence labeling of reactive Cys thiols was performed as described earlier.
Mass spectrometry
A model chemical cystine (Cys–Cys) was treated with NaHS or GSH at concentrations of 0.1, 1, and 10 mM at room temperature for 1 h and loaded onto a mass spectrometer (Agilent 1100 series LC/MSD DAD, Santa Clara, CA). Mass spectrometry analysis used a drying gas flow of 10 L·min−1, a nebulizer pressure of 35 psig, a drying gas temperature of 350°C, and a capillary voltage of 3000 V (negative). Data acquisition and analysis were performed using Agilent LC-MS software.
Construction of mutant human EGFR (C797A)
The mutant form of EGFR protein, in which Cys797 was replaced with an alanine (C797A), was provided by SignalChem according to a custom-development program. Two DNA primers (P-1, EGFR-F2329-BglII, sequence 5′-AAGATCTAGTGGAGAAGCTCCCAA and P-2, EGFR-R3879-NotI, sequence 5′-AGCGGCCGCTCATGCTCCAATAAATTCACTGC) were used for PCR amplification of human EGFR (695-end) cDNA. Two other primers (P-3, EGFRc797a-F2626, sequence 5′-CCCTTCGGCGCCCTCCTGGACTATGTCCG and P-4, EGFRc797a-R2647, sequence 5′-AGTCCAGGAGGGCGCCGAAGGGCATGAGCTG) were used to generate mutant EGFR (C797A).
Two pieces of mutant cDNA were separately generated using primer pairs P-1/P-4 and P-2/P-3 with wild-type EGFR cDNA. Then, these two PCR products were used to amplify the mutant EGFR (C797A) product with the primer pair P-1/P-2. After sequencing validation, mutant EGFR (C797A) cDNA was subcloned into a glutathione S-transferase (GST)-tag pSP1G4T2 expression plasmid. The purified pSP1G4T2-EGFR (C797A) plasmid was cotransfected with linear AcNPV DNA (BD PharMingen, Franklin Lakes, NJ) into Spodoptera frugiperda (Sf9) cells to produce recombinant baculoviruses, which were amplified to a high viral titer in Sf9 cells. Large-scale protein expression of the recombinant EGFR (C797A) baculovirus was induced by infecting Sf9 insect cells. Expression was under the control of the pH promoter and proceeded for ∼3 days in Sf9 cells. These cells were lysed, and the expressed GST-EGFR (C797A) proteins were purified using affinity chromatography with a GSH–agarose column. The eluted proteins were subjected to SDS-PAGE analysis and had a purity of ∼80%. To determine EGFR (C797A) kinase activity, serial dilution assays were performed using a substrate of Poly Glu:Tyr (4:1) and 33P-labeled ATP (Custom development report; SignalChem).
Rat EGFR (C798A) mutant overexpression
The EGFR mutant at Cys798 to Ala798 was cloned into a lentiviral vector expression plasmid LV4 (GenePharma, Shanghai, China). The experimental procedures used were as follows. Four DNA primers (P-1, sequence 5′-GATAGCGGCCGCGCCACCATGCGACCCTCAGGGACTGCGAGA; P-2, sequence 5′-AGTCCAGGAGGGCACCATAGGGCATGAGTTGTGTAAT; P-3, sequence 5′-CCTATGGTGCCCTCCTGGACTATGTCC-GAGAACATAAGGA; P-4, sequence 5′-GATCCGTCTCGGATCCTCATGCTCCACTAAACTCACTGCTTGGCGGTG) were used for PCR amplification of rat EGFR (C798A) cDNA. Two pieces of mutant cDNA were generated separately using primer pairs P-1/P-2 and P-3/P-4 with wild-type EGFR cDNA. Then, these two PCR products were used to amplify the mutant EGFR (C798A) product with the primer pair P-1/P-4. Mutant EGFR (C798A) cDNA was digested with NotI and Esp3I, and the lentiviral LV4 vector was digested with NotI and BamHI. Then, digested mutant EGFR (C798A) cDNA was connected to the linearized lentiviral vector with T4 DNA ligase.
The ligation products were transformed in competent cells and singled out as positive clones. After sequencing validation, a rat EGFR (C798A) mutant lentivirus was obtained (custom service report; GenePharma). Renal tubular epithelial cells grown to 40%–60% confluence were infected with the rat EGFR (C798A) mutant lentivirus or control lentivirus. The multiplicity of infection of lentivirus transfection was 75. These cells were passaged for further experiments after infection for 3 days.
Experimental protocol for chronic salt-loaded rats
Male SD rats weighing 140–160 g were used. The DOCA-salt hypertensive rat model was established as the chronic salt-loaded rat model. A week after nephrectomy, rats were randomly divided into six groups. Groups 1 and 2 were sham groups that were subcutaneously injected with oil once every week, intraperitoneally injected with (i) vehicle or (ii) NaHS (10 μmol·kg−1·day−1) for 4 weeks, and fed rat chow and normal water. Groups 3 and 4 were control groups that were subcutaneously injected with DOCA (30 mg·kg−1) once every week, intraperitoneally injected with either (iii) vehicle or (iv) NaHS (10 μmol·kg−1·day−1) for 4 weeks, and fed rat chow with 1% NaCl+0.2% KCl in their drinking water. Groups 5 and 6 were erlotinib groups that were subcutaneously injected with DOCA (30 mg·kg−1) once every week, intraperitoneally injected with either (v) vehicle or (vi) NaHS (10 μmol·kg−1·day−1) and erlotinib (10 mg·kg−1·day−1) for 4 weeks, and fed rat chow with 1% NaCl+0.2% KCl in their drinking water.
A 24-h urine sample was collected once every week using a metabolic cage and assayed for sodium, potassium, chlorine, and creatinine concentrations. After 4 weeks, rats were anesthetized with chloral hydrate (300 mg·kg−1), and polyethylene tubes were inserted into their left carotid arteries and kept in place for a second day to measure arterial pressure while the rats were conscious. Blood samples were collected for the measurement of plasma antidiuretic hormone (ADH), aldosterone (ALD), ouabain, creatinine, and H2S levels and other biochemical profiles. Kidneys were weighed and divided into two parts; one was used for isolating renal tubular epithelial cells to determinate Na+/K+-ATPase enzymatic activity, while the other was used for the determination of c-Src, EGFR, gab1, PI3K, and Akt phosphorylation; EGFR expression; and ouabain levels. Right thigh muscles were weighed and heated in a 60°C oven for 24 h, after which they were weighed and used to determine muscle water content. The GFR was expressed as the Ccr: Ccr=U×V/P, where U=urinary creatinine concentration, V=urinary flow rate, and P=plasma creatinine concentration.
Urinary sodium concentrations: Totally, 300 μl of six antimony potassium hydroxide and 200 μl of accelerator were added to a 20-μl sample. After the solution became turbid, 1 ml of ethanol was added and mixed well. Absorbance was read at 620 nm with a microplate reader.
Urinary potassium concentrations: A protein precipitant (0.9 ml) was added to 0.1 ml of a urine sample and centrifuged at 1370 g for 5 min. Then, 200 μl of sodium tetraphenylborate was added to 50 μl of the supernatant. After incubation for 5 min, absorbance was read at 450 nm with a microplate reader.
Urinary chlorine concentrations: Totally, 250 μl of mercuric thiocyanate was added to 10 μl of a urine sample and mixed well. After incubation for 5 min, absorbance was read at 480 nm with a microplate reader.
Urinary creatinine concentrations: A urine sample was diluted to 1:200 with double-distilled water. Then, 50 μl of picric acid and 50 μl of 0.75 M NaOH were added and mixed well. After 10 min in a water bath at 37°C, absorbance was read at 510 nm with a microplate reader.
Plasma H2S concentrations: To a 1.5-ml tube that contained 0.25 ml of 1% zinc acetate and 0.4 ml of distilled water, 0.1 ml of plasma was added. Then, 0.133 ml of 20 mM N,N-dimethyl-p-phenylenediamine dihydrochloride was added in 7.2 M HCl and 0.133 ml of 30 mM FeCl3 in 1.2 M HCl. The sample was incubated at room temperature for 20 min. Then, 0.25 ml of 10% trichloroacetic acid was added and centrifuged at 4000 g for 5 min. Absorbance at 670 nm was read with a microplate reader. H2S concentrations were determined from a standard curve.
Rat hemodynamic and renal function determinations
Male SD rats weighing 250–300 g were used for hemodynamic and renal function determinations as previously described (27). A rat was intraperitoneally anesthetized with 14% urethane (5 ml·kg−1) and placed on a thermostatically controlled table to maintain body temperature at 37°C. Polyethylene tubes were inserted into the right femoral vein and left carotid artery for intravenous infusions and for measurements of arterial pressure and MAP (MFLab200 computerized recording system, Department of Physiology and Pathophysiology, Shanghai Medical College, Fudan University, Shanghai, China). Through a dorsal incision, a transit-time flow meter probe (Transonic Systems, Inc., Ithaca, NY) was placed around the left renal artery to measure RBF. A catheter was implanted in the bladder to collect urine. Saline (0.9%) was intravenously infused at the rate of 1.5 ml·h−1 throughout this procedure. After 1 h for equilibration and baseline measurements, 20 μmol·kg−1 NaHS or vehicle was injected continuously for 1 min via a femoral vein. Hemodynamic parameters were recorded for 90 min, and urine was collected every 30 min. Urine samples were used for sodium, potassium, and chlorine determinations as described earlier. UNaV, UKV, and UClV were derived from their respective concentrations multiplied by UV.
Acute salt-loaded rat hemodynamic and renal function determinations
Male SD rats weighing 250–300 g were used for surgery as described earlier. To determine the GFR, 0.9% saline containing 2% inulin was infused intravenously at the rate of 1.5 ml·h−1 throughout the procedure. After 1 h for equilibration and baseline measurements, 5% NaCl (6.6 ml·kg−1) was injected continuously for 1 h via a femoral vein to establish an acute salt-loaded rat model. Hemodynamic parameters were recorded for 60 min, and urine was collected every 30 min. Blood samples (200 μl) were obtained midway during each 30 min of urine collection. Then, different concentrations of NaHS (0, 5, 10, 20, 30, 60, and 90 μmol·kg−1) were injected continuously for 1 min via a femoral vein. Hemodynamic parameters were recorded for 90 min, and urine and blood samples were collected as noted earlier. Urine samples were used to determine sodium, potassium, and chlorine concentrations as described earlier. Blood samples were centrifuged at 1370 g for 15 min at 4°C. Urine and blood inulin concentrations were determined with the anthrone-sulphuric acid colorimetric method. A standard curve of concentration versus absorbance was used to determine sample concentrations. GFR was expressed as inulin clearance: C=U×V/P, where U=urinary inulin concentration, V=urinary flow rate, and P=plasma inulin concentration. ADH and ALD levels were determined by radioimmunoassay kits (The Second Military Medical University, Shanghai, China; Ruijin Hospital, Shanghai Jiaotong University, China).
Statistical analysis
Results are expressed as means±standard errors. Statistical analyses were performed using SPSS Statistics 17.0 (SPSS, Inc., Chicago, IL). The results for three or more groups were compared using one-way ANOVA. Comparisons between two groups were made using Student's t-test. All group data were tested for normal distributions before statistical comparisons. p<0.05 was considered statistically significant.
Supplementary Material
Abbreviations Used
- ADH
antidiuretic hormone
- ALD
aldosterone
- ANOVA
analysis of variance
- BCECF
the acetoxymethyl ester of 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein
- Ccr
creatinine clearance rate
- CV
control vector
- Cy3
Cy3 maleimide
- Cy5
Cy5 maleimide
- Cys
cysteine
- DHE
dihydroethidium
- DMEM/F12
Dulbecco's modified Eagle's medium/Ham's nutrient mixture F12
- DN-Akt
dominant-negative mutant of Akt
- DOCA
deoxycorticosterone acetate
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EIPA
5-(N-ethyl-N-isopropyl)-amiloride
- FBS
fetal bovine serum
- GFR
glomerular filtration rate
- GSH
glutathione
- GST
glutathione S-transferase
- HA
hemagglutinin
- H2S
hydrogen sulfide
- MAP
mean arterial pressure
- Na+/K+-ATPase inhibitor
a positive control
- NEM
N-ethylmaleimide
- NHE3
sodium hydrogen exchanger-3
- NIH
National Institutes of Health
- Oua
ouabain
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PDB
protein data bank
- PVDF
polyvinyl difluoride
- RBF
renal blood flow
- ROS
reactive oxygen species
- SD
Sprague–Dawley
- SDS
sodium dodecyl sulfate
- siRNA
small interfering RNA
- T-AOC
total antioxidant capacity
- UClV
urinary chlorine excretion
- UKV
urinary potassium excretion
- UNaV
urinary sodium excretion
- UV
urinary flow rate
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology (2010CB912601 and 2012ZX09501001) of China, the National Natural Science Foundation of China (81230003, 81000045, and 81000939), the 50th China Postdoctoral Science Foundation funded project (2011M500728), the Ministry of Education (20110071130009), and the Science and Technology Commission of Shanghai Municipality (12JC1400700), and by a key laboratory program of the Education Commission of Shanghai Municipality (ZDSYS14005).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bertorello AM, Aperia A, Walaas SI, Nairn AC, and Greengard P. Phosphorylation of the catalytic subunit of Na+, K+-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A 88: 11359–11362, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, and Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, and Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, and Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Czernik AJ. and Petrack B. Somatostatin receptor binding in rat cerebral cortex. J Biol Chem 258: 5525–5530, 1983 [PubMed] [Google Scholar]
- 6.Efendiev R, Bertorello AM, Pressley TA, Rousselot M, Feraille E, and Pedemonte CH. Simultaneous phosphorylation of Ser11 and Ser18 in the alpha-subunit promotes the recruitment of Na(+),K(+)-ATPase molecules to the plasma membrane. Biochemistry 39: 9884–9892, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, Du J, and Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318: 756–763, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Go GW. and Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med 85: 19–28, 2012 [PMC free article] [PubMed] [Google Scholar]
- 9.Guyton AC. Blood pressure control-special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Hosoki R, Matsuki N, and Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Jaitovich A. and Bertorello AM. Salt, Na+, K+-ATPase and hypertension. Life Sci 86: 73–78, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee SH, Koh YJ, Koh GY, Chio C, Yu DY, Kim J, and Kang SW. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell 44: 545–558, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Kirley TL. Inactivation of (Na+,K+)-ATPase by beta-mercaptoethanol. Differential sensitivity to reduction of the three beta subunit disulfide bonds. J Biol Chem 265: 4227–4232, 1990 [PubMed] [Google Scholar]
- 14.Knepper MA. and Brooks HL. Regulation of the sodium transporters NHE3, NKCC2 and NCC in the kidney. Curr Opin Nephrol Hypertens 10: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lemmon MA. and Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J. Ouabain-induced endocytosis and signal transduction of the Na/K-ATPase. Front Biosci 10: 2056–2063, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, and Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int 66: 227–241, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Liang M, Liu L, Malhotra D, Xie Z, and Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int 67: 1844–1854, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Liu J. and Shapiro JI. Regulation of sodium pump endocytosis by cardiotonic steroids: molecular mechanisms and physiological implications. Pathophysiology 14: 171–181, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lurje G. and Lenz HJ. EGFR signaling and drug discovery. Oncology 77: 400–410, 2009 [DOI] [PubMed] [Google Scholar]
- 21.McKee M, Scavone C, and Nathanson JA. Nitric oxide, cGMP, and hormone regulation of active sodium transport. Proc Natl Acad Sci U S A 91: 12056–12060, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muth TR, Gottardi CJ, Roush DL, and Caplan MJ. A basolateral sorting signal is encoded in the alpha-subunit of Na-K-ATPase. Am J Physiol 274: 688–696, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, and Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 190: 133–157, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, and Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues GA, Falasca M, Zhang Z, Ong SH, and Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 20: 1448–1459, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 51: 660–672, 1949 [Google Scholar]
- 27.Shi Y, Cao YX, Lu N, Yao T, and Zhu YC. Hemodynamic-independent anti-natriuretic effect of urotensin II in spontaneously hypertensive rats. Peptides 29: 783–794, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Zhang JN, Zhao D, Wang QS, Gu YC, Ma HP, and Zhang ZR. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin 32: 789–797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, and Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhu Y, and Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, Kometiani P, Tillekeratne M, Jin R, and Xie Z. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem 284: 14921–14929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong TH. and Carroll KS. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry 51: 9954–9965, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, and Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li XH, Chen Y, Zhu JH, Ding YJ, Liu J, and Zhu YC. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal 19: 5–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao LL, Huang XW, Wang YG, Cao YX, Zhang CC, and Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. Am J Physiol Heart Circ Physiol 298: H1310–H1319, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, and Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A 97: 6556–6561, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Jiang G, Zhao A, Bondeva T, Hirszel P, and Balla T. Inhibition of Na,K-ATPase activates PI3 kinase and inhibits apoptosis in LLC-PK1 cells. Biochem Biophys Res Commun 285: 46–51, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.